 If you suffered Tasigna side effects, contact a Tasigna lawyer today for a free case evaluation If you suffered Tasigna side effects, contact a Tasigna lawyer today for a free case evaluation First approved in 2007, Tasigna (nilotinib), is a prescription medication used to treat chronic myeloid leukaemia (CML). Tasigna is part of a class of drugs known as tyrosine kinase inhibitors (TKIs). Other TKI drugs include Gleevec (imatinib), also made by Novartis, and Sprycel (dasatinib), made by Bristol-Myers Squibb. These drugs help treat leukemia by blocking the protein tyrosine kinases—a protein that stimulates the growth of cancer cells. In blocking this protein, TKI drugs like Tasigna help stop leukemia from spreading. Tasigna is prescribed as a twice daily drug and is taken orally in capsule form. If you suffered Tasigna side effects, including Tasigna cardiovascular side effects, call Tasigna lawyer Timothy L. Miles today for a free case evaluation and see if you qualify for a Tasigna lawsuit. If you are eligible for a Tasigna lawsuit, you could be entitled to significant compensation. Read on to see if you may be eligible for a Tasignant lawsuit. WHAT IS THE PROBLEM WITH TASIGNA?Injured patients and their families are now filing lawsuits against Tasigna manufacturer Novartis, on the grounds that the company failed to warn of the drug’s cardiovascular risks. Studies have allegedly linked the drug to serious cardiovascular side effects like Long QT syndrome, a condition involving irregular heartbeats, and atherosclerosis, a disease that causes plaque to build up in the arteries. The FDA issued a black box warning (the highest warning they can issue) for Tasigna’s link to QT interval prolongation and sudden death. WHAT ARE THE TASIGNA CARDIOVASCULAR SIDE EFFECTS?To date, approximately 15,500 Tasigna related adverse events and 2,786 deaths have allegedly been reported to the FDA to date. These include serious Tasigna side effects including cardiovascular side effects, like Long QT syndrome, myocardial ischemia, and atherosclerosis. Tasigna has one black box warning—the highest warning the FDA can issue—for Long QT syndrome, or QT interval prolongation, and sudden death. It is a condition that affects heart rhythm, resulting in fast, irregular heartbeats. If left untreated, it can result in fainting, seizures, and sudden death. Tasigna may also been linked to myocardial ischemia, a condition involving reduced blood flow to the heart, causing a decreased amount of oxygen sent to the heart. Myocardial ischemia is usually caused by a blockage of the coronary arteries. Recent lawsuits have also been filed over the drug’s alleged connection to atherosclerosis, a disease that causes fatty deposits to build up and eventually clog arteries. This serious condition gradually reduces blood flow and oxygen to cells. Atherosclerosis is currently the leading cause of death in the developed world. It may eventually cause the following complications:
ARE THERE STUDIES LINKING TASIGNA TO CARDIOVASCULAR EVENTS?Two recent studies have connected Tasigna with cardiovascular events:
The FDA’s adverse event reporting database has received more than 100 reports of Tasigna-related myocardial ischemia diagnoses. Myocardial ischemia is a condition that is usually caused by a blockage of the coronary arteries. It results in reduced blood flow to the heart, decreasing the amount of oxygen to the heart. In an FDA post-market review, 5.8% of patients taking Tasigna allegedly suffered ischemic heart. Atherosclerosis is a disease that causes fatty deposits to build up and eventually clog arteries. This serious condition gradually reduces blood flow and oxygen to cells. Atherosclerosis may eventually cause coronary artery disease, peripheral arterial disease, blood clots, heart attacks, or strokes. It’s currently the leading cause of death in the developed world. Since 2013, Tasigna products sold in Canada come with a warning of the drug’s connection to atherosclerosis. This label change came after Canadian health officials warned that 277 cases of atherosclerosis were allegedly reported worldwide. Lawsuits allege that Novartis never warned of the risk of atherosclerosis in the U.S. though, despite these reports. Other serious Tasigna side effects noticed by the FDA that may be linked to Tasigna include low blood cell counts; decreased blood flow to the heart, lungs, or brain; pancreatitis; and Tumor Lysis Syndrome, a condition that can occur when cancer cells are broken down quickly, resulting in kidney failure or an abnormal heartbeat. Lawsuits have been filed some patients against Novartis. who took Tasigna and subsequently suffered a heart or circulation problem. The lawsuits allege Novartis failed to properly warn of these side effects. WHAT ARE SOME OF THE TASIGNA SIDE EFFECTS?Novartis warns of the following Tasigna side effects:
HOW DO I KNOW IF I AM ELIGIBLE FOR A TASIGNA LAWSUIT?If you or a loved one took Tasigna and suffered heart or circulation problems (including complications like blocked arteries, heart attacks, or strokes), you could be eligible for a Tasigna lawsuit and possible entitled to substantial compensation. Call Tasigna lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Tasigna lawsuit. IF I AM ELIGIBLE FOR A TASIGNA LAWSUIT, HOW MUCH CAN I GET OUT OF A TASIGNA LAWSUIT?If you are eligible for a Tasigna lawsuit and are successful, you can recover money for some of the following damages:
Broadly speaking, a plaintiff in a Tasigna lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they qualify for a Tasigna lawsuit and are successful. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT, KEEP ALL YOUR TASIGNA DOCUMENTATIONMaintaining all your Tasigna documentation and other evidence is critical if you believe you are eligible for a Tasigna lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Tasigna lawsuit. Your Tasigna lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Tasigna documents if you believe you are eligible for a Tasigna lawsuit, including:
CALL A TASIGNA LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT?If you or a loved one took Tasigna and suffered heart or circulation problems (including complications like blocked arteries, heart attacks, or strokes), you could be eligible for a Tasigna Lawsuit. If you suffered Tasigna side effects, including Tasigna cardiovascular side effects, call Tasigna lawyer Timothy L. Miles today for a free case evaluation and see if you qualify for a Tasigna lawsuit. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, securities fraud, civil procedure, corporate formation, corporate takeover litigation, derivative lawsuits, and more. Visit out website.  Call an Actemra lawyer today if you suffered serious Actemra side effects Call an Actemra lawyer today if you suffered serious Actemra side effects Roche’s Actemra was heralded as a breakthrough therapy for rheumatoid arthritis when it was introduced in 2010. The drug’s website proclaims that “Actemra works differently” and shows a patient happily scuba diving and walking on a tropical beach. But nowhere does Roche mention certain side effects that may have contributed to more than 1,100 patient deaths. Research suggests that Actemra patients, compared to those taking competing rheumatoid arthritis drugs, face similar risks for heart attacks, heart failure, strokes, lung disease, and pancreatitis. But unlike the competition, Actemra’s label does not warn about these side effects. If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation. WHAT ARE THE ACTEMRA SIDE EFFECTS TO THE HEART AND LUNGS?A STAT News report and postmarketing studies raise questions about the adequacy of Actemra's safety warnings. STAT reviewed more than 500,000 adverse event reports on leading rheumatoid arthritis drugs, including Actemra, Humira, and Remicade. STAT found evidence that Roche downplayed Actemra’s cardiovascular, pancreatic, and lung side effects. According to STAT, from 2010 to 2016 the U.S. Food and Drug Administration (FDA) logged more than 13,500 Actemra adverse event reports, including 1,128 patients who died after taking Actemra. While safety data indicate the risks of heart attacks, strokes, heart failure, and interstitial lung disease are as high or higher for Actemra patients than for patients taking some similar drugs, Actemra’s warning label—unlike its competition's—does not reflect these risks. For example, Actemra patients reported more cases of interstitial lung disease than Remicade patients, and nearly the same number as Humira patients. But while Remicade and Humira have interstitial lung disease warnings, Actemra does not. STAT’s findings are supported by postmarketing studies—clinical studies conducted after a pharmaceutical product goes on the market to better understand drug risks. The FDA ordered Actemra postmarketing studies because premarket testing revealed potentially worrisome side effects. One such study compared Actemra to another arthritis drug, Enbrel, and found rates of stroke and heart failure were about 1.5 times higher in Actemra users. Enbrel has strong warnings for patients with cardiovascular disease. Actemra does not have similar warnings despite similar or worse cardiovascular risks. The takeaway from these findings is that doctors and patients may not be getting a complete picture of Actemra’s risk-benefit profile. DO DRUG MANUFACTURERS HAVE A DUTY TO WARN?Pharmaceutical products are not expected to be completely without harmful side effects. But if a drugmaker understands that a drug presents a certain risk, it has a duty to put an appropriate warning label on the product. That way patients and their doctors can evaluate all known risks and benefits and make an educated decision about whether a medication is right for them. While the FDA has authority to demand warning labels, the responsibility ultimately falls on drug manufacturers. Actemra has garnered FDA attention since it was approved in 2010. At one point the agency considered a pancreatitis warning label. But Genetech/Roche talked the FDA out of the warning. The agency then approved broader use of Actemra for giant cell arteritis. Conflicts of interest between manufacturers and regulators plague the drug safety system. The STAT report details how all 11 authors of an Actemra postmarketing study had financial ties to Roche or Genetech. And a former FDA manager who oversaw Actemra’s approval for rheumatoid arthritis now works for Roche, where he helps gain FDA approval for new uses of the drug. The drug safety system, in other words, cannot always be trusted to put patient safety first. That’s why, when patients suffer harm from drug side effects, they often must turn to the legal system. HOW DO I KNOW IF I AM ELIGIBLE FOR AN ACTEMRA LAWSUIT?We are looking into claims that Genetech/Roche failed to warn about the following Actemra side effects:
Actemra patients who suffered any of these Actemra side effects may be eligible for an Actemra lawsuit. IF I MEET THE REQUIREMENTS FOR AN ACTEMRA LAWSUIT, WHAT CAN I GET?If you meet the requirements for an Actemra lawsuit and are successful you can recover money for some of the following damages: • Past and future medical bills (including medication, hospital stays, and in-home care) • Pain and suffering • Lost wages • Loss of earning capacity • Funeral expenses (in the event of a loved one’s death) Broadly speaking, a plaintiff who is eligible for an Actemra lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they meet the requirements for an Actemra lawsuit and are successful. HOW MUCH DOES IT COST TO HIRE AN ACTEMRA LAWYER IF I MEET THE REQUIREMENTS FOR AN ACTEMRA LAWSUIT?It does not cost anything to hire an Actemra lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case. An Actemra lawyer can explain the process and answer any questions you may have including whether you are eligible for an Actemra lawsuit. CALL AN ACTEMRA LAWYER TODAY IF YOU SUFFERED SERIOUS ACTEMRA SIDE EFFECTS?If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L. Miles today to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation. timothy l. milesTimothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, securities fraud, civil procedure, corporate formation, corporate takeover litigation, derivative lawsuits, and more. Visit out website.  If you suffered Kombiglyze XR side effects, call a Kombiglyze XR lawyer today If you suffered Kombiglyze XR side effects, call a Kombiglyze XR lawyer today Kombiglyze XR is a combination of metformin (a biguanide) and saxagliptin (a DPP4 inhibitor). Kombiglyze XR works by lowering the amount of sugar made by your liver, decreasing the amount of sugar absorbed by your intestine, increasing the amount of insulin your body makes, and helps your body respond better to insulin. However, research shows that type 2 diabetes drug Kombiglyze XR (saxagliptin and metformin) may cause heart failure or other cardiovascular injuries in some patients. According to the Centers for Disease Control and Prevention (CDC), 30 million Americans have diabetes and 84 million have pre-diabetes. Metformin—one of the drugs found in combination in Kombiglyze—is among the top 10 most popular prescription drugs taken by Americans. Analysts also predict that Onglyza (saxagliptin), Kombiglyze’s sister drug, could reach $1.8 billion in sales in 2018. In 2015, roughly 386,000 people were prescribed Onglyza or Kombiglyze XR, the U.S. Food and Drug Administration (FDA) reported. Given that so many people take these drugs, any side effects they may cause that are severe enough to hospitalize patients should be treated with concern. Dozens of patients are filing lawsuits against Kombiglyze’s manufacturer AstraZeneca and its previous partner Bristol-Myers Squibb alleging that the companies failed to adequately test the drugs’ safety prior to selling it, which ultimately caused their cardiovascular injuries. If you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR side effects including heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation. Read on for answers to five frequently asked questions about a Kombiglyze lawsuit and see if you are eligible for a Kombiglyze XR lawsuit. WHAT ARE THE KOMBIGLYZE XR SIDE EFFECTS?The following are some of the common Kombiglyze XR side effects:
Additionally, dozens of lawsuits allege other Kombiglyze XR side effects including serious cardiovascular injury. If you suffered serious Kombiglyze XR side effects call Kombiglyze lawyer Timothy L. Miles today to see if you are eligible for a Kombiglyze XR lawsuit. WHAT IS THE PROBLEM WITH KOMBIGLYZE XR?Research shows Kombiglyze XR and other similar drugs may put patients at risk of heart complications. Dozens of patients are filing lawsuits against Kombiglyze’s manufacturer AstraZeneca and its previous partner Bristol-Myers Squibb alleging that the companies failed to adequately test the drugs’ safety prior to selling it, which ultimately caused their cardiovascular injuries. If you suffered cardiovascular injuries after taking Kombiglyze or other serious Kombiglyze XR side effects call Kombiglyze lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Kombiglyze XR lawsuit. DOES KOMBIGLYZE XR PUT PATIENTS AT RISK OF HEART FAILURE?A clinical trial funded by AstraZeneca and Bristol-Myers Squibb was one of the first studies to report cardiovascular health risks associated with these drugs. Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus, or the SAVOR trial, found that patients with a history of heart problems who took Onglyza or Kombiglyze XR had a 27% greater chance of being hospitalized for heart failure than patients who took a placebo. Patients with chronic kidney disease and / or previous heart failure were more likely to be hospitalized. When an FDA panel reviewed the results from the SAVOR study, 14 out of 15 panelists voted to require a safety warning on all Onglyza and Kombiglyze XR bottles that the drugs could result in cardiovascular injury. Patients are filing lawsuits against AstraZeneca and Bristol-Myers Squibb alleging that Kombiglyze XR caused their cardiovascular injuries. Complaints allege that AstraZeneca and Bristol-Myers Squibb failed to adequately test the drug prior to their sale. In February 2018, more than 80 Onglyza and Kombiglyze XR lawsuits were centralized in Lexington, Kentucky. Plaintiffs' attorneys said that centralization is key to reducing inconsistencies since these lawsuits were "just the tip of the iceberg" and the number of filed complaints was expected to grow. In a complaint filed in January 2018, Illinois plaintiff Jerald Adams alleged that after being exposed to saxagliptin after taking both Onglyza and Kombiglyze XR, the diagnosis of his heart failure was “directly related to taking these drugs.” In the complaint, Mr. Adams alleged that the manufacturers not only knew of the cardiovascular injury risk associated with saxagliptin, but that they also failed to warn or mitigate this risk despite their knowledge. "At all relevant times, defendants had knowledge that there was a significantly increased risk of adverse events associated with saxagliptin, including heart failure, congestive heart failure, cardiac failure, and death related to those events, and despite this knowledge, defendants continued to manufacture, market, distribute, sell, and profit from sales of saxagliptin," the complaint alleges. Some lawsuits filed in New Jersey claim that Bristol-Myers and AstraZeneca did not just do a poor job of testing the safety of their drugs, but that they also did not conduct the clinical trials recommended by the FDA in 2008 prior to Onglyza’s sale debut in 2009. In 2008, the FDA Endocrinologic and Metabolic Drugs Advisory Committee "determined that concerns about cardiovascular risk should be more thoroughly addressed during drug development." Bristol-Myers and AstraZeneca failed to do so and began marketing Onglyza in 2009. WHAT DO THE KOMBIGLYZE XR LAWSUITS ALLEGE?Kombiglyze XR lawsuits allege that AstraZeneca and Bristol-Myers Squibb failed to:
HOW DO I KNOW IF I AM ELIGIBLE OR A KOMBIGLYZE XR LAWSUIT?If you or a loved one took Kombiglyze XR as directed and suffered from heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, you may meet the requirements for a Kombiglyze XR lawsuit and possibly be entitled to substantial compensation. Call a Kombiglyze XR lawyer for free today who can tell you if you are eligible for a Kombiglyze XR lawsuit. IF YOU SUFFERED FROM KOMBIGLYZE XR SIDE EFFECTS, CALL A KOMBIGLYZE XR LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A KOMBIGLYZE XR LAWSUITIf you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR Side Effects, specifically: heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, securities fraud, civil procedure, corporate formation, corporate takeover litigation, derivative lawsuits, and more. Visit out website.  Call an Onglyza lawyer if you took Onglyza and suffered serious side effects Call an Onglyza lawyer if you took Onglyza and suffered serious side effects Onglyza (saxagliptin) and Kombiglyze XR (saxagliptin and metformin) belong to a new class of diabetic drugs known as DPP-4 inhibitors. Onglyza gained federal drug approval by the U.S. Food & Drug Administration (FDA) despite initial concerns over the cardiovascular safety of the drug. These concerns were echoed in a post-marketing safety study that found Onglyza may be associated with an increased risk of heart failure and an increased risk of death. If you or a loved one took Onglyza as directed and suffered from Onglyza side effects including heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Onglyza therapy, contact Onglyza lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for an Onglyza lawsuit and possibly entitled to substantial compensation. Read on for answers to five frequently asked questions about the Onglyza lawsuit. WHAT IS ONGLYZA?Onglyza (saxagliptin) is an add-on medication to diet and exercise to improve blood sugar control in people with type 2 diabetes. It is a is a dipeptidyl peptidase-4 (DPP-4) inhibitor that increases the amount of insulin your body makes. Onglyza comes in tablets in dosages of 2.5 mg and 5 mg taken once by mouth daily. WHO SHOULD NOT TAKE ONGLYZA?You should not take Onglyza if you are allergic to any of its ingredients. Serious allergic reactions can occur with Onglyza including swelling of the face, throat, lips and difficulty breathing, among others. Your doctor should test your blood to measure how well your kidneys work before and during treatment with Onglyza. You may need a lower dose of Onglyza if your kidneys are not working well. WHAT IS THE PROBLEM WITH ONGLYZA?Type 2 diabetes patients have a significantly increased risk of cardiovascular complications, and the majority of diabetics die from such complications. Amid growing concerns over the safety of many diabetes drugs, in 2008 the FDA recommended that the makers of new type 2 diabetes drugs provide evidence that the drugs do not increase the risk of cardiovascular events such as heart attacks. Onglyza met the FDA's cardiovascular safety criteria. Though the FDA approved the new diabetic drug, it had reservations about the sufficiency of Onglyza clinical trials and ordered AstraZeneca to perform a post-marketing study. As a result, AstraZeneca completed a 16,000-patient cardiovascular outcomes trial called SAVOR. The results of that trial were published in the New England Journal of Medicine and raised major Onglyza safety concerns. SAVOR found that Onglyza patients were 27% more likely to be hospitalized for heart failure. The study also found a significant increase in the rate of “all-cause mortality” among Onglyza patients. The warning signs of heart failure include:
Following the release of SAVOR, the FDA issued an Onglyza drug safety communication and convened an advisory committee meeting to analyze the results and recommend further action. The committee voted 14-to-1 to add information to Onglyza’s label warning about heart failure risks. The other member voted to withdraw Onglyza from the U.S. market. The committee, however, did not advise updating the warning label to address the measured increase in all-cause mortality among Onglyza patients and did not recommend any restrictions on prescribing the drug. In an April 2016 drug safety communication, the FDA announced that Onglyza and Kombiglyze XR are now required to carry warnings about heart failure risk. Other FDA warnings about saxagliptin include:
WHAT ARE THE ONGLYZA SIDE EFFECTS?In addition to heart failure, Onglyza is linked to these adverse side effects:
HOW DO I KNOW IF I MEET THE REQUIREMENTS FOR AN ONGLYZA LAWSUIT?If you or a loved one took Onglyza as directed and suffered suffered heart failure, death, or another serious cardiovascular injury, after starting Onglyza therapy, you may meet the requirements for an Onglyza lawsuit and possibly be entitled to substantial compensation. Call a Onglyza lawyer for free today who can tell you if you are eligible for an Onglyza lawsuit. CONTACT AN ONGLYZA LAWYER TO SEE IF YOU ARE ELIGIBLE FOR AN ONGLYZA LAWSUIT?If you or a loved one took Onglyza as directed and suffered from Onglyza Side Effects, specifically: heart failure, death or another serious cardiovascular injury —such as congestive heart failure or myocardial infarction, after starting Onglyza therapy, contact Onglyza lawyer Timothy L. Miles today to see if you are eligible for a Onglyza lawsuit and possibly entitled to substantial compensation. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, securities fraud, civil procedure, corporate formation, corporate takeover litigation, derivative lawsuits, and more. Visit out website.
If you took Byetta and developed serious Byetta side effects, contact Byetta Lawyer Timothy L. Miles today about a Byetta Lawsuit
Byetta (exenatide) was the first of a new class drugs used to treat type 2 diabetes drugs known as glucagon-like peptide 1 (GLP-1) receptor agonists which generated a lot of early excitement with added benefits including a low risk of hypoglycemia and successful weight loss. However, early optimism was halted by reports that Byetta and similar drugs were a possible risk factor for pancreatitis and pancreatic cancer.
There have been hundreds of pancreatic cancer lawsuits filed against the manufacturers of Byetta, Victoza, Janument, and Januvia. These lawsuits were all transferred the Southern District of California by the Judicial Panel on Multidistrict Litigation for coordinated and pretrial discovery and proceedings. A ruling by the trial judge in 2015 appeared to end the case until a recent court of appeals decision in favor of the Byetta plaintiffs holding their claims may proceed in federal court. If you or a loved one took Byetta and were diagnosed with pancreatic cancer, contact Byetta lawsuit lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for Byetta lawsuit and possible entitled to substantial damages. (855) Tim-M-Law (855-846-6529). Read on to learn what you need to know about the Byetta side effects. WHAT IS BYETTA? Contact a Byetta lawyer today if you took Byetta and developed pancreatic cancer Contact a Byetta lawyer today if you took Byetta and developed pancreatic cancer
Byetta (exenatide) is a medicine used to treat type 2 diabetes. It is glucagon-like peptide-1 (GLP-1) receptor agonist. A receptor agonist drug means that it binds to a certain receptor and causes the same action as the substance that normally binds to that receptor. Byetta, by binding to and activating the GLP-1 receptor, tells your body to release more insulin, stops the body from releasing more sugar, and slows down digestion.
Byetta is a solution for injection available in pre‑filled pens of 5mcg or 10 mcg. The shots are given by the patient twice a day by injection under the skin in the abdomen, thigh or upper arm. The starting dose of Byetta is 5 mcg, twice a day before a meal. After four weeks, the dose can be increased to 10 mcg depending on how well your blood sugar levels respond to Byetta injections. WHAT IS THE PROBLEM WITH BYETTA?
Incretin mimetic drugs such as Byetta regulate the production of incretin, a type of hormone that stimulates the release of insulin in the body. Byetta does this by injecting patients with a substance that mimics a particular incretin called Glucagon-Like Peptide-1 (GLP-1). Byetta lawsuits allege this process overstimulates the pancreas causing cells in the pancreas to multiply resulting in pancreatic cancer.
BYETTA SIDE EFFECTS
Byetta side effects include, among others:
A more severe allergic reaction to Byetta is rare but possible. The symptoms of a severe allergic reaction can include:
IS THERE A LINK BETWEEN BYETTA AND PANCREATIC CANCER?
A number of scientific studies support plaintiff claims that Byetta causes pancreatic cancer.
WHAT ARE THE SYMPTOMS OF PANCREATITIS?
Pancreatitis, an inflammation of the pancreas, can cause the following symptoms, among others:
It is not known for certain if Byetta or other similar incretin mimetic diabetes drugs can cause pancreatic cancer. However, Incretin mimetics treat type 2 diabetes by mimicking the action of GLP-1, a hormone that stimulates the pancreas to produce more insulin. In certain people, this could possibly cause chronic inflammation of the pancreas, also known as pancreatitis, which is a known risk factor for pancreatic cancer. HOW DO I KNOW IF I AM ELIGIBLE FOR A BYETTA LAWSUIT?
If you meet the following criteria, you may be eligible for a Byetta lawsuit:
Plaintiffs may also seek claims related to the wrongful death of a loved one who was prescribed Byetta and developed fatal pancreatic cancer. IF I AM ELIGIBLE FOR A BYETTA LAWSUIT, HOW MUCH DOES IT COST TO HIRE A BYETTA LAWYER?
It does not cost anything to hire a Byetta lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case. A Byetta lawyer can explain the process and answer any questions you may have including whether you are eligible for a Byetta lawsuit.
WHAT IS A BYETTA LAWSUIT?
A Byetta Lawsuit is a demand from the defendants for monetary compensation by patients who took Byetta and subsequently developed serious Byetta Side Effects. The lawsuits allege the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. If you suffered Byetta Side Effects and are eligible for a Byetta lawsuit, you may possibly be entitled to substantial compensation. A Byetta Lawsuit Lawyer can answer these questions and is available anytime just give us a call or submit this form for a free case evaluation to see if you are eligible for a Byetta lawsuit.
IF I AM ELIGIBLE FOR A BYETTA LAWSUIT, WHAT CAN A BYETTA LAWSUIT LAWYER DO FOR ME?
Because of the complexity of the cases and the need to hire experts, a products liability attorney is the go-to attorney when you have been injured by a drug or a product that is defective like Byetta. A Byetta Lawsuit Lawyer, who is an experienced products liability attorney, can help you by putting you in the best possible position to win your Byetta Lawsuit and recover the most compensation if you are eligible for a Byetta lawsuit.
IF I AM ELIGIBLE FOR A BYETTA LAWSUIT, HOW LONG WILL A BYETTA LAWSUIT TAKE?
While every case is different depending on the unique facts of the case, most mass tort cases take at least a year to settle, with others may take two, three, or more years. Byetta lawsuit settlements can vary in time based on many different factors including:
An extraordinarily large number of lawsuits
Call a Byetta Lawsuit Lawyer today who can answer this question based on the unique facts of your case. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A BYETTA LAWSUIT, KEEP ALL YOUR BYETTA DOCUMENTATION
Maintaining all your Byetta documentation and other evidence is critical if you believe you are eligible for a Byetta lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Byetta Lawsuit. Your Byetta lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Byetta documents if you believe you are eligible for a Byetta lawsuit, including:
CALL A BYETTA LAWSUIT LAWYER IF YOU TOOK BYETTA AND WERE DIAGNOSED WITH PANCREATIC CANCER
If you or a loved one took Byetta and were diagnosed with pancreatic cancer, you may be eligible to file a Byetta lawsuit. Contact Byetta lawsuit lawyer Timothy L. Miles today and see if you meet the requirements for a Byetta lawsuit and could be eligible for substantial compensation. (855) Tim-M-Law (855-846-6529) or [email protected].
TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, securities fraud, civil procedure, corporate formation, corporate takeover litigation, derivative lawsuits, and more. Visit out website.  If you took Merida as directed and developed Merida side effects call Merida lawyer Timothy L. Miles If you took Merida as directed and developed Merida side effects call Merida lawyer Timothy L. Miles Meridia, made by Abbott Laboratories, was approved in 1997 for weight loss and weight loss maintenance for obese patients, and also very obese individuals who already had other health risk factors for cardiovascular disease. Meridia was approved after clinical studies showed that more patients taking Meridia lost at least 5 percent of their body weight than members of the placebo group who relied solely on diet and exercise. However, the U.S. Food & Drug Administration (FDA) has now requested that Meridia be recalled following a nearly year-long safety review of the drug which was initiated after researchers published the results of a study known as SCOUT (Sibutramine Cardiovascular Outcomes Trial). The study, which prompted a Meridia recall in Europe, showed a 16% percent increase in the risk of serious Meridia side effects, including:
SCOUT monitored 10,000 obese patients over the age of 55 who had a history of heart disease, and type 2 diabetes or similar risk factors. The initial purpose of the study was to show that weight loss through Meridia use lowered the risk of heart problems. However, that was not the case. In fact, the results suggested that those taking the weight loss drug had an increased chance of suffering Meridia cardiovascular side effects. Doctors have been advised to stop prescribing Meridia and begin discussing weight loss alternatives with their patients. If you or a loved one suffered serious Meridia side effects including a heart attack, stroke or even death, contact Meridia lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial compensation. Read on to see if you may be eligible for a Meridia lawsuit. SERIOUS MERIDIA SIDE EFFECTSIn the beginning, Meridia seemed to be a booming success as it was used by millions of individuals around the world. However, hundreds of users began reporting serious Meridia side effects including:
Patients taking Meridia have also experienced:
Additionally, approximately 30 deaths were caused by Merida in the United States. Researchers looked at 10,000 patients who were obese, over the age of 55 with a history of risk factors and found that 11.4% of patients using Meridia suffered heart problems, compared to 10% given a sugar pill. As of June 30, 2009, the FDA Adverse Event Reactions (AERS) database indicates that at least 84 Meridia deaths from cardiovascular causes have been reported to the FDA.' If you suffered severe Meridia side effects call Meridia Lawyer Timothy L. Miles for a free case evaluation to see if you are eligible for a Meridia lawsuit. IF I AM ELIGIBLE FOR A MERIDIA LAWSUIT, WHAT CAN I GET OUT OF A MERIDIA LAWSUIT?If you meet the requirements for a Meridia lawsuit and are successful you can recover money for some of the following damages: • Past and future medical bills (including medication, hospital stays, and in-home care) • Pain and suffering • Lost wages • Loss of earning capacity • Funeral expenses (in the event of a loved one’s death) Broadly speaking, a plaintiff who is eligible for a Meridia lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they meet the requirements for a Meridia lawsuit and are successful. Call today to see if you are eligible for a Meridia lawsuit. HOW MUCH DOES IT COST TO HIRE A MERIDA LAWYER IF I MEET THE REQUIREMENTS FOR A MERIDA LAWSUITIt does not cost anything to hire a Meridia lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case. A Meridia lawyer can explain the process and answer any questions you may have including whether you are eligible for a Meridia lawsuit. So, call today and see if you meet the requirements for a Meridia lawsuit. If eligible for a Meridia lawsuit, you may be entitled to substantial compensation as a result of suffering Meridia side effects. WHAT IS A MERIDA LAWSUIT?A Meridia Lawsuit is a demand from the defendants for monetary compensation by patients who took Meridia and subsequently developed Meridia Side Effects including a heart attack, stroke or even death. The lawsuits allege the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. If you suffered Meridia Side Effects and are eligible a Meridia lawsuit, you may possibly be entitled to substantial compensation. A Meridia Lawyer can answer these questions and is available anytime just give us a call or submit the form below for a free case evaluation to see if you are eligible for a Meridia lawsuit. WHAT CAN A MERIDIA LAWYER DO FOR ME IF I AM ELIGIBLE FOR A MERIDIA LAWSUIT?Because of the complexity of the cases and the need to hire experts, a products liability attorney is the go-to attorney when you have been injured by a drug or a product that is defective like Meridia and are eligible for a Meridia lawsuit. A Meridia Lawyer, who is an experienced products liability attorney, can help you by putting you in the best possible position to win your Meridia Lawsuit and recover the most compensation. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A MERIDIA LAWSUIT, KEEP ALL YOUR MERIDIA DOCUMENTATIONMaintaining all your Meridia documentation and other evidence is critical if you believe you are eligible for a Meridia lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Meridia lawsuit. Your Meridia lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Meridia documents if you believe you are eligible for a Meridia lawsuit, including:
IF YOU SUFFERED MERIDIA SIDE EFFECTS, CALL A MERIDIA LAWYER TODAY TO SEE IF YOU ARE ELIGIBLE FOR A MERIDIA LAWSUITIf you or a loved one suffered serious Meridia side effects including a heart attack, stroke or even death, contact Meridia lawyer Timothy L. Miles today to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial compensation. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today and see if you meet the requirements for a Meridia lawsuit.
TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Call an Actemra lawyer today if you suffered serious Actemra side effects Call an Actemra lawyer today if you suffered serious Actemra side effects Roche’s Actemra was heralded as a breakthrough therapy for rheumatoid arthritis when it was introduced in 2010. The drug’s website proclaims that “Actemra works differently” and shows a patient happily scuba diving and walking on a tropical beach. But nowhere does Roche mention certain side effects that may have contributed to more than 1,100 patient deaths. Research suggests that Actemra patients, compared to those taking competing rheumatoid arthritis drugs, face similar risks for heart attacks, heart failure, strokes, lung disease, and pancreatitis. But unlike the competition, Actemra’s label does not warn about of these Actemra side effects. If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation. HOW DO I KNOW IF I AM ELIGIBLE FOR AN ACTEMRA LAWSUIT?We are looking into claims that Genetech/Roche failed to warn about the following Actemra side effects:
Actemra patients who suffered any of these Actemra side effects may be eligible for an Actemra lawsuit. IF I MEET THE REQUIREMENTS FOR AN ACTEMRA LAWSUIT, WHAT CAN I GET?If you suffered Actemra side effects and you meet the requirements for an Actemra lawsuit and are successful you can recover money for some of the following damages: • Past and future medical bills (including medication, hospital stays, and in-home care) • Pain and suffering • Lost wages • Loss of earning capacity • Funeral expenses (in the event of a loved one’s death) Broadly speaking, a plaintiff who suffered Actemra side effects and therefore is eligible for an Actemra lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they meet the requirements for an Actemra lawsuit and are successful. HOW MUCH DOES IT COST TO HIRE AN ACTEMRA LAWYER IF I MEET THE REQUIREMENTS FOR AN ACTEMRA LAWSUIT?It does not cost anything to hire a Actemra lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case. An Actemra lawyer can explain the process and answer any questions you may have including whether you are eligible for an Actemra lawsuit as a result of your suffering Actemra side effects. IF I AM ELIGIBLE FOR AN ACTEMRA LAWSUIT, HOW DOES AN ACTEMRA LAWSUIT WORK?Your Actemra lawyer will meet with you to ask you some questions to confirm you are eligible for an Actemra lawsuit, including your medical history and the Actemra side effects you suffered and how they effected your life. Your Actemra Lawyer will then draft what is known as a complaint. This will be a multi-page document explaining why the defendant is responsible for your injuries and what damages (that is, compensation) you are seeking. Once your complaint is filed with the court, your Actemra Lawsuit officially begins. From here, it will be a lot of back and forth between your Actemra lawyer and the attorney(s) for the defendant in an attempt to resolve the matter. The attorneys may take review documents, take depositions, issue subpoenas, hire experts, calculate damages, attend hearings, and file motions, briefs, evidence or other documents with the court during the stages of the lawsuit. If your Actemra lawsuit is not dismissed and a settlement cannot be reached, the case will proceed to a jury trial. IF I AM ELIGIBLE FOR AN ACTEMRA LAWSUIT, WHAT CAN AN ACTEMRA LAWYER DO FOR ME?Because of the complexity of the cases and the need to hire experts, a products liability attorney is the go-to attorney when you have been injured by a drug or a product that is defective like Actemra. An Actemra Lawyer, who is an experienced products liability attorney, can help you by putting you in the best possible position to win your Actemra Lawsuit and recover the most compensation. WHAT IS AN ACTEMRA LAWSUIT?An Actemra Lawsuit is a demand from the defendants for monetary compensation by patients who took Actemra and suffered heart, lung, or pancreatic side effects while taking the drug. The lawsuit would allege the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. If you are eligible an Actemra lawsuit and possibly be entitled to substantial compensation. An Actemra Lawyer can answer these questions including whether you meet the requirements for an Actemra lawsuit and is available anytime just give us a call for a free case evaluation. (855) 846-6529 CALL AN ACTEMRA LAWYER TODAY IF YOU SUFFERED SERIOUS ACTEMRA SIDE EFFECTS?If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L. Miles today to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation.
TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Call a Kombiglyze XR lawyer if you suffered Kombiglyze XR side effects Call a Kombiglyze XR lawyer if you suffered Kombiglyze XR side effects Kombiglyze XR is a combination of metformin (a biguanide) and saxagliptin (a DPP4 inhibitor). Kombiglyze XR works by lowering the amount of sugar made by your liver, decreasing the amount of sugar absorbed by your intestine, increasing the amount of insulin your body makes, and helps your body respond better to insulin. However, research shows that type 2 diabetes drug Kombiglyze XR (saxagliptin and metformin) may cause heart failure or other cardiovascular injuries in some patients. According to the Centers for Disease Control and Prevention (CDC), 30 million Americans have diabetes and 84 million have pre-diabetes. Metformin—one of the drugs found in combination in Kombiglyze—is among the top 10 most popular prescription drugs taken by Americans. Analysts also predict that Onglyza (saxagliptin), Kombiglyze’s sister drug, could reach $1.8 billion in sales in 2018. In 2015, roughly 386,000 people were prescribed Onglyza or Kombiglyze XR, the U.S. Food and Drug Administration (FDA) reported. Given that so many people take these drugs, any side effects they may cause that are severe enough to hospitalize patients should be treated with concern. Dozens of patients are filing lawsuits against Kombiglyze’s manufacturer AstraZeneca and its previous partner Bristol-Myers Squibb alleging that the companies failed to adequately test the drugs’ safety prior to selling it, which ultimately caused their cardiovascular injuries. If you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR side effects including heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation. Read on to learn more about the documentation and other evidence you should maintain if you suffered serious Kombiglyze side effects and believe you are you are eligible for a Kombiglyze lawsuit. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A KOMBIGLYZE XR LAWSUIT, KEEP ALL YOUR KOMBIGLYZE XR DOCUMENTATION If you suffered Kombiglyze XR side effects, call a Kombiglyze XR lawyer today If you suffered Kombiglyze XR side effects, call a Kombiglyze XR lawyer today Maintaining all your Kombiglyze XR documentation and other evidence is critical if you believe you are eligible for a Kombiglyze XR lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Kombiglyze XR lawsuit. Your Kombiglyze XR lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Kombiglyze XR documents if you believe you are eligible for a Kombiglyze XR lawsuit, including:
IF YOU SUFFERED FROM KOMBIGLYZE XR SIDE EFFECTS, CALL A KOMBIGLYZE XR LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A KOMBIGLYZE XR LAWSUITIf you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR Side Effects, specifically: heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation as a result of suffering Kombiglyze XR side effects.
TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Call an Elmiron lawyer about an Elmiron lawsuit if you suffered Elmiron Eye Damage Call an Elmiron lawyer about an Elmiron lawsuit if you suffered Elmiron Eye Damage The Firm is reviewing claims on behalf of Interstitial Cystitis (IC) patients who took the prescription drug Elmiron and now may be suffering from a severe eye disease called maculopathy or has suffered vision impairment as a result. If you developed malculopathy or a vision impairment and took Elmiron, or have additional questions, give Elmiron Lawyer Timothy L. Miles a call today for a free case evaluation, you could be entitled to significantly compensation if you meet the Elmiron Lawsuit Criteria and file an Elmiron lawsuit. Get started by filling out a free case evaluation form or calling us. (855) Tim-M-Law (855-846-6529) Read on to see if you may be eligible for an Elmiron lawsuit. WHAT IS ELMIRON?Since 1996, millions of patients have been prescribed Elmiron in order to treat their interstitial cystitis (IC), otherwise known as painful bladder syndrome. But there is now reason to believe that this drug may cause a serious eye condition in those taking it, one that may result in blindness. This condition is a form of maculopathy – the degeneration of the macula, the central part of the retina. Maculopathy’s first symptoms are blurred vision, dark spots, trouble focusing, and other impairments: as it progresses, it may lead to total blindness. As the evidence has piled up, the manufacturer of Elmiron – Janssen Pharmaceuticals – has failed to warn the public about these risks. Millions of people are taking a drug that may one day rob them of their vision, and an untold number may have already suffered serious health conditions. Elmiron eye damage lawsuits on behalf of the injured have already begun claiming their maculopathy was caused by Elmiron. If you or a loved one took Elmiron and has since been diagnosed with maculopathy, or vision impairment you may be eligible for compensation. Contact an Elmiron Lawyer today to have your case reviewed for free and see if you meet the Elmiron lawsuit criteria and are eligible for an Elmiron lawsuit. WHAT ARE THE SYMPTOMS OF ELMIRON RELATED RETINAL MACULOPATHY?A newly discovered condition has come to light, and it is presently unknown how many symptoms there actually are, or how serious this can end up being for those suffering from it. The currently known symptoms associated with this eye condition include:
HOW DO I GET STARTED IF I SUFFERED ELMIRON EYE DAMAGE AND BELIEVE I AM ELIGIBLE FOR AN ELMIRON LAWSUIT?If you suffered Elmiron Eye Damage fill out and submit this form for a Free Case Evaluation with an Elmiron Lawyer to see if you are eligible for a Elmiron lawsuit and possibly entitled to substantial compensation. WHY SHOULD I FILE AN ELMIRON EYE DAMAGE LAWSUIT?If you or a loved one suffered Elmiron Eye Damage, you have a right to file a claim for compensation for your pain and suffering and other damages such as lost wages, hospital and medical bills. An Elmiron lawyer will investigate your claim free of charge to determine if you are eligible for an Elmiron lawsuit. WHAT IS A ELMIRON LAWSUIT?An Elmiron Lawsuit is a demand from the defendants for monetary compensation by patients who took Elmiron and subsequently developed Elmiron Eye Damage. The lawsuits allege the defendants made a defective drug and failed to warn physicians about its serious side effects including Elmiron Eye Damage. If you suffered Elmiron Eye Damage and are eligible for an Elmiron lawsuit, you may possibly be entitled to substantial compensation. An Elmiron Lawyer can answer these questions and is available anytime just give us a call or for a free case evaluation to see if you are eligible for an Elmiron lawsuit. IF I AM ELIGIBLE FOR AN ELMIRON LAWSUIT, HOW MUCH COMPENSATION CAN I GET IN A ELMIRON LAWSUIT?If you are eligible for an Elmiron lawsuit, a successful plaintiff is entitled to compensatory damages which would include compensation for things such as:
If your case proceeds to trial it is also possible the court could impose punitive damages, intended to punish the defendant for their actions. IF I AM ELIGIBLE FOR AN ELMIRON LAWSUIT, HOW MUCH DOES IT COST TO HIRE AN ELMIRON LAWYER?It does not cost anything to hire an Elmiron lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case, so contact us today. An Elmiron lawyer can explain the process and answer any questions you may have. So, the call is free and so is the fee unless we win your case. If you suffered Elmiron Eye damage, contact us today for a free case evaluation, you may be eligible for an Elmiron lawsuit and possibly entitled to substantial compensation. CALL AN ELMIRON LAWYER TODAY TO SEE IF YOU ARE ELIGIBLE FOR AN ELMIRON LAWSUIT?If you were diagnosed with maculopathy or vision impairment caused by Elmiron, contact an Elmiron Lawyer today as you most likely meet the Elmiron lawsuit criteria and are eligible for an Elmiron lawsuit and possibly recover substantial compensation. The call is free and so is the fee unless we will you case. So, call today and see what an Elmiron lawyer can do for you.
So, give us a call today or submit the free case evaluation form and see if you and are eligible for an Elmiron lawsuit. TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  If you suffered Kombiglyze XR side effects, call a Kombiglyxe XR lawyer today If you suffered Kombiglyze XR side effects, call a Kombiglyxe XR lawyer today Kombiglyze XR is a combination of metformin (a biguanide) and saxagliptin (a DPP4 inhibitor). Kombiglyze XR works by lowering the amount of sugar made by your liver, decreasing the amount of sugar absorbed by your intestine, increasing the amount of insulin your body makes, and helps your body respond better to insulin. However, research shows that type 2 diabetes drug Kombiglyze XR (saxagliptin and metformin) may cause heart failure or other cardiovascular injuries in some patients. According to the Centers for Disease Control and Prevention (CDC), 30 million Americans have diabetes and 84 million have pre-diabetes. Metformin—one of the drugs found in combination in Kombiglyze—is among the top 10 most popular prescription drugs taken by Americans. Analysts also predict that Onglyza (saxagliptin), Kombiglyze’s sister drug, could reach $1.8 billion in sales in 2018. In 2015, roughly 386,000 people were prescribed Onglyza or Kombiglyze XR, the U.S. Food and Drug Administration (FDA) reported. Given that so many people take these drugs, any side effects they may cause that are severe enough to hospitalize patients should be treated with concern. Dozens of patients are filing lawsuits against Kombiglyze’s manufacturer AstraZeneca and its previous partner Bristol-Myers Squibb alleging that the companies failed to adequately test the drugs’ safety prior to selling it, which ultimately caused their cardiovascular injuries. If you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR side effects including heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation. Read on to learn more about a Koxmiglyze XR lawsuit. WHAT IS A KOMBIGLYZE XR LAWSUIT?A Kombiglyze XR Lawsuit is a demand from the defendants for monetary compensation by patients who took Kombiglyze XR and subsequently developed Kombiglyze XR Side Effects including cardiovascular injuries. The lawsuits allege the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. If you suffered Kombiglyze XR Side Effects and are eligible a Kombiglyze XR lawsuit, you may possibly be entitled to substantial compensation. A Kombiglyze XR Lawyer can answer these questions and is available anytime just give us a call or submit the form below for a free case evaluation to see if you are eligible for a Kombiglyze XR lawsuit. HOW DO I KNOW IF I AM ELIGIBLE OR A KOMBIGLYZE XR LAWSUIT?If you or a loved one took Kombiglyze XR as directed and suffered from heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, you may meet the requirements for a Kombiglyze XR lawsuit and possibly be entitled to substantial compensation. Call a Kombiglyze XR lawyer for free today who can tell you if you are eligible for a Kombiglyze XR lawsuit. IF I MEET THE REQUIREMENTS FOR A KOMBIGLYZE XR LAWSUIT, WHAT CAN I GET?If you meet the requirements for a Kombiglyze XR lawsuit and are successful you can recover money for some of the following damages: • Past and future medical bills (including medication, hospital stays, and in-home care) • Pain and suffering • Lost wages • Loss of earning capacity • Funeral expenses (in the event of a loved one’s death) Broadly speaking, a plaintiff who is eligible for a Kombiglyze XR lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they meet the requirements for a Kombiglyze XR lawsuit and are successful. IF I AM ELIGIBLE FOR A KOMBIGLYZE XR LAWSUIT, HOW MUCH DOES IT COST TO HIRE A KOMBIGLYZE XR LAWYER?It does not cost anything to hire a Kombiglyze XR lawyer. We take all cases on a contingency basis which means we do not get paid unless we win your case. A Kombiglyze XR lawyer can explain the process and answer any questions you may have including whether you are eligible for a Kombiglyze XR lawsuit. WHAT CAN A KOMBIGLYZE XR LAWYER DO FOR ME IF I AM ELIGIBLE FOR A KOMBIGLYZE XR LAWSUIT ?Because of the complexity of the cases and the need to hire experts, a products liability attorney is the go-to attorney when you have been injured by a drug or a product that is defective like Kombiglyze XR. A Kombiglyze XR Lawyer, who is an experienced products liability attorney, can help you by putting you in the best possible position to win your Kombiglyze XR Lawsuit and recover the most compensation if you are eligible for a Kombiglyze XR lawsuit. HOW DOES A KOMBIGLYZE XR LAWSUIT WORK?If you meet the requirements for a Kombiglyze XR lawsuit, before it is filed with the court, your Kombiglyze XR Lawsuit Lawyer will fully explain the process and will then need to ask you a few questions concerning the circumstances of you diagnoses, how long you have been taking Kombiglyze XR and your medical history. Your Kombiglyze XR Lawyer will then draft what is known as a complaint. This will be a multi-page document explaining why the defendant is responsible for your injuries and what damages (that is, compensation) you are seeking. Once your complaint is filed with the court, your Kombiglyze XR Lawsuit officially begins. From here, it will be a lot of back and forth between your Kombiglyze XR lawyer and the attorney(s) for the defendant in an attempt to resolve the matter. The attorneys may take review documents, take depositions, issue subpoenas, hire experts, calculate damages, attend hearings, and file motions, briefs, evidence or other documents with the court during the stages of the lawsuit. If your Kombiglyze XR lawsuit is not dismissed and a settlement cannot be reached, the case will proceed to a jury trial. IF I MEET THE REQUIREMENTS FOR A KOMBIGLYZE XR LAWSUIT, HOW LONG WILL A KOMBIGLYZE XR LAWSUIT TAKE?While every case is different depending on the unique facts of the case, most mass tort cases take at least a year to settle, with others may take two, three, or more years. A settlement time can vary based on many different factors including:
Call a Kombiglyze XR Lawyer today who can answer this question based on the unique facts of your case. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A KOMBIGLYZE XR LAWSUIT, KEEP ALL YOUR KOMBIGLYZE XR DOCUMENTATIONMaintaining all your Kombiglyze XR documentation and other evidence is critical if you believe you are eligible for a Kombiglyze XR lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Kombiglyze XR lawsuit. Your Kombiglyze XR lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Kombiglyze XR documents if you believe you are eligible for a Kombiglyze XR lawsuit, including:
IF YOU SUFFERED FROM KOMBIGLYZE XR SIDE EFFECTS, CALL A KOMBIGLYZE XR LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A KOMBIGLYZE XR LAWSUITIf you or a loved one took Kombiglyze XR as directed and suffered from Kombiglyze XR Side Effects, specifically: heart failure or other cardiovascular injuries—such as congestive heart failure or myocardial infarction, after starting Kombiglyze XR therapy, contact Kombiglyze XR lawyer Timothy L. Miles today to see if you are eligible for a Kombiglyze XR lawsuit and possibly entitled to substantial compensation.
TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Contact a Victoza Cancer Lawyer today if you took Victoza and were diagnosed with pancreatic cancer Contact a Victoza Cancer Lawyer today if you took Victoza and were diagnosed with pancreatic cancer Hundreds of Victoza cancer lawsuits filed by victims from across the country allege that Victoza, which is an incretin mimetic drug used to treat type 2 diabetes, may cause pancreatic cancer and that the manufacturer of Victoza, Novo Nordisk, failed to warn them of this risk. Other incretin mimetic drugs have also been linked to this dangerous side effect. As a result, Januvia, Janumet, and Byetta have also been linked to pancreatic cancer. Manufacturers Novo Nordisk, Merck & Co., Eli Lilly, and Amylin Pharmaceuticals now hundreds of lawsuits that have been coordinated for pretrial proceedings in a multidistrict litigation (MDL) in Southern California while further research continues on the question of does Victoza cause pancreatic cancer continues. If you or a loved one took Victoza were later diagnosed with pancreatic cancer, contact Victoza Cancer Lawyer Timothy L. Miles today for a free case evaluation and see if you are eligible for a Victoza cancer lawsuit and possibly entitled to substantial compensation. WHAT KIND OF DRUG IS VICTOZA?Victoza (liraglutide) is a medicine used to treat type 2 diabetes. It is glucagon-like peptide-1 (GLP-1) receptor agonist. A receptor agonist drug means that it binds to a certain receptor and causes the same action as the substance that normally binds to that receptor. Victoza, by binding to and activating the GLP-1 receptor, tells your body to release more insulin, stops the body from releasing more sugar, and slows down digestion. Victoza is a solution for injection available in pre‑filled pens. The shots are given by the patient once a day by injection under the skin in the abdomen, thigh or upper arm. The starting dose of Victoza is 0.6 mg, and after at least one week, the dose is then increased to 1.2 mg. In certain patients, the dose can be further increased to 1.8 mg one week later, if necessary, achieve better control of blood glucose. WHAT IS THE PROBLEM WITH VICTOZA? Contact Victoza lawyer Timothy L. Miles if you suffered Victoza side effects Contact Victoza lawyer Timothy L. Miles if you suffered Victoza side effects Incretin mimetic drugs such as Victoza regulate the production of incretin, a type of hormone that stimulates the release of insulin in the body. Victoza does this by injecting patients with a substance that mimics a particular incretin called Glucagon-Like Peptide-1 (GLP-1). Victoza cancer lawsuits allege this process overstimulates the pancreas causing cells in the pancreas to multiply resulting in pancreatic cancer. Researchers suspect that the process used by Victoza, and other incretin mimetic drugs may overstimulate the pancreas, and in some cases, cause pancreas cells to multiply. One expert in this area, Dr. Peter Butler, who is a diabetes expert at UCLA, discovered a growth of precancerous lesions in the pancreas when this process occurred. Additionally, according to the U.S. Food & Drug Administration's (FDA) adverse event database, patients taking Januvia, a similar drug to Victoza, are 10 times more likely to be diagnosed with pancreatic cancer. Beyond these observations though, the research is still unclear if and how incretin mimetic drugs cause pancreatic cancer. In 2013, the FDA requested that researchers investigate the correlation in greater detail. However, as more evidence is gathered for a more detailed review on the question of does Victoza cause pancreatic cancer, patients are continuing to file lawsuits asserting they got pancreatic cancer after taking their type 2 diabetes medication. In fact, over 2,500 Victoza patients have registered complaints with the FDA also asserting that the medication caused them to have pancreatic side effects. The vast majority (more than 2,300) allege pancreatitis, both acute and chronic. Moreover, similar reports have been made for other incretin mimetics: Roughly 2,400 Januvia patients and more than 3,000 Byetta patients have similarly complained of pancreatitis and filed lawsuits with claims similar to the Victoza cancer lawsuits. Finally, the American Cancer Society warns that patients with chronic pancreatitis (inflammation of the pancreas) may have an increased risk of developing pancreatic cancer. If you or a loved one suffered the side effects of Victoza, and were later diagnosed with pancreatic cancer, contact Victoza Cancer Lawyer Timothy L. Miles today and see if you are eligible for a Victoza cancer lawsuit. WHAT IS THE STATUS OF THE VICTOZA CANCER LAWSUITS?The Judicial Panel on Multidistrict Litigation (MDL) ordered that 749 incretin mimetic cases filed against leading manufacturers Novo Nordisk, Merck & Co., and Eli Lilly & Co be coordinated for pretrial proceedings in one multi-district litigation (MDL) and transferred the MDL cases to the Southern District of California and assigned to Judge Anthony Battaglia. The Victoza cancer lawsuits, all allege the drugs cause pancreatic cancer and that the manufacturers failed to warn doctors and patients of the risk. In 2015, Federal Battaglia dismissed the then 744 lawsuits alleging a failure-to-warn in favor of the defendants' argument that the FDA would have prevented them from putting a pancreatic cancer warning on their drug labels, and since the FDA is a federal agency, it overrides state laws. In September 2016, plaintiffs asserted that Judge Battaglia had misinterpreted federal preemption law. In December 2017, a Ninth Circuit judicial panel agreed, and ruled in favor of plaintiffs, claiming that Judge Battaglia misinterpreted the Supreme Court ruling in Buckman Co. v. Plaintiffs' Legal Committee which states that private parties cannot pursue state law tort claim alleging violations of the Food, Drug, and Cosmetic Act (FDCA). The court of appeals also ruled that consumers claimed defendants violated the common law duty to warn, which falls outside the FDCA. WHAT DO THE VICTOZA CANCER LAWSUITS ALLLEGE?The Victoza Cancer Lawsuits allege a combination of the following claims:
HOW DO I KNOW IF I AM ELIGIBLE FOR A VICTOZA CANCER LAWSUIT?If you or a loved one took Victoza and were diagnosed with pancreatic cancer in 2005 or later, or suffered other serious Victoza side effects, you may be eligible to file a Victoza cancer lawsuit against Novo Nordisk and may be entitled to substantial compensation. CALL A VICTOZA CANCER LAWYER AND SEE IF YOU ARE ELIGIBLE FOR A VICTOZA CANCER LAWSUIT?If you or a loved one took Victoza and were diagnosed with pancreatic cancer in 2005 or later, or suffered other serious Victoza side effects you may be eligible for a Victoza cancer lawsuit. Contact Victoza cancer lawyer Timothy L. Miles today and see if you qualify for a Victoza cancer lawsuit and could be eligible for substantial compensation. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today.
TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Call an Onglyza lawyer if you suffered severe Onglyza side effects Call an Onglyza lawyer if you suffered severe Onglyza side effects Onglyza (saxagliptin) and Kombiglyze XR (saxagliptin and metformin) belong to a new class of diabetic drugs known as DPP-4 inhibitors. Onglyza gained federal drug approval by the U.S. Food & Drug Administration (FDA) despite initial concerns over the cardiovascular safety of the drug. These concerns were echoed in a post-marketing safety study that found Onglyza may be associated with an increased risk of heart failure and an increased risk of death. If you or a loved one took Onglyza as directed and suffered from Onglyza Side Effects, specifically: heart failure, death or another serious cardiovascular injury —such as congestive heart failure or myocardial infarction, after starting Onglyza therapy, contact Onglyza lawyer Timothy L. Miles today to see if you are eligible for a Onglyza lawsuit and possibly entitled to substantial compensation. Read on for the answers to five frequently asked questions about an Onglyza lawsuit. WHAT IS ONGLYZA?Onglyza (saxagliptin) is an add-on medication to diet and exercise to improve blood sugar control in people with type 2 diabetes. It is a is a dipeptidyl peptidase-4 (DPP-4) inhibitor that increases the amount of insulin your body makes. Onglyza comes in tablets in dosages of 2.5 mg and 5 mg taken once by mouth daily. WHO SHOULD NOT TAKE ONGLYZA?You should not take Onglyza if you are allergic to any of its ingredients. Serious allergic reactions can occur with Onglyza including swelling of the face, throat, lips and difficulty breathing, among others. Your doctor should test your blood to measure how well your kidneys work before and during treatment with Onglyza. You may need a lower dose of Onglyza if your kidneys are not working well. WHAT IS THE PROBLEM WITH ONGLYZA?Type 2 diabetes patients have a significantly increased risk of cardiovascular complications, and the majority of diabetics die from such complications. Amid growing concerns over the safety of many diabetes drugs, in 2008 the FDA recommended that the makers of new type 2 diabetes drugs provide evidence that the drugs do not increase the risk of cardiovascular events such as heart attacks. Onglyza met the FDA's cardiovascular safety criteria. Though the FDA approved the new diabetic drug, it had reservations about the sufficiency of Onglyza clinical trials and ordered AstraZeneca to perform a post-marketing study. As a result, AstraZeneca completed a 16,000-patient cardiovascular outcomes trial called SAVOR. The results of that trial were published in the New England Journal of Medicine and raised major Onglyza safety concerns. SAVOR found that Onglyza patients were 27% more likely to be hospitalized for heart failure. The study also found a significant increase in the rate of “all-cause mortality” among Onglyza patients. The warning signs of heart failure include:
Following the release of SAVOR, the FDA issued an Onglyza drug safety communication and convened an advisory committee meeting to analyze the results and recommend further action. The committee voted 14-to-1 to add information to Onglyza’s label warning about heart failure risks. The other member voted to withdraw Onglyza from the U.S. market. The committee, however, did not advise updating the warning label to address the measured increase in all-cause mortality among Onglyza patients and did not recommend any restrictions on prescribing the drug. In an April 2016 drug safety communication, the FDA announced that Onglyza and Kombiglyze XR are now required to carry warnings about heart failure risk. Other FDA warnings about saxagliptin include:
WHAT ARE THE ONGLYZA SIDE EFFECTS?In addition to heart failure, Onglyza is linked to these adverse side effects:
HOW DO I KNOW IF I MEET THE REQUIREMENTS FOR AN ONGLYZA LAWSUIT?If you or a loved one took Onglyza as directed and suffered suffered Onglyza side effects; specifically, heart failure, death, or another serious cardiovascular injury, after starting Onglyza therapy, you may meet the requirements for an Onglyza lawsuit and possibly be entitled to substantial compensation. Call a Onglyza lawyer for free today who can tell you if you are eligible for an Onglyza lawsuit. CONTACT AN ONGLYZA LAWYER TO SEE IF YOU ARE ELIGIBLE FOR AN ONGLYZA LAWSUIT?If you or a loved one took Onglyza as directed and suffered from Onglyza Side Effects, specifically: heart failure, death or another serious cardiovascular injury —such as congestive heart failure or myocardial infarction, after starting Onglyza therapy, contact Onglyza lawyer Timothy L. Miles today to see if you are eligible for a Onglyza lawsuit and possibly entitled to substantial compensation. TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A VICTOZA CANCER LAWSUIT, KEEP ALL YOUR VICTOZA DOCUMENTATION4/24/2023
 Contact a Victoza cancer lawyer today if you took Victoza and were diagnosed with pancreatic cancer Contact a Victoza cancer lawyer today if you took Victoza and were diagnosed with pancreatic cancer Hundreds of Victoza cancer lawsuits filed by victims from across the country allege that Victoza, which is an incretin mimetic drug used to treat type 2 diabetes, may cause pancreatic cancer and that the manufacturer of Victoza, Novo Nordisk, failed to warn them of this risk. Other incretin mimetic drugs have also been linked to this dangerous side effect. As a result, Januvia, Janumet, and Byetta have also been linked to pancreatic cancer. Manufacturers Novo Nordisk, Merck & Co., Eli Lilly, and Amylin Pharmaceuticals now hundreds of lawsuits that have been coordinated for pretrial proceedings in a multidistrict litigation (MDL) in Southern California while further research continues on the question of does Victoza cause pancreatic cancer continues. If you or a loved one took Victoza were later diagnosed with pancreatic cancer, contact Victoza Cancer Lawyer Timothy L. Miles today for a free case evaluation and see if you are eligible for a Victoza cancer lawsuit and possibly entitled to substantial compensation. If you or a loved one took Victoza and suffered serious Victoza side effects and believe are you are eligible for a Victoza cancer lawsuit it is vital that you maintain all you Victoza documentation and other evidence in your possession. You will need this evidence to prove your case and that you are entitled to compensation in a Victoza cancer lawsuit as a result of suffering serious Victoza side effects. Read on to learn more about the documentation and other evidence you should maintain if you suffered serious Victoza side effects and believe you are you are eligible for a Victoza cancer lawsuit. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A VICTOZA CANCER LAWSUIT, KEEP ALL YOUR VICTOZA DOCUMENTATIONMaintaining all your Victoza documentation and other evidence is critical if you believe you are eligible for a Victoza cancer lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Victoza cancer lawsuit. Your Victoza lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Victoza documents if you believe you are eligible for a Victoza cancer lawsuit, including:
CALL A VICTOZA CANCER LAWYER AND SEE IF YOU ARE ELIGIBLE FOR A VICTOZA CANCER LAWSUIT?If you or a loved one took Victoza and were diagnosed with pancreatic cancer in 2005 or later, or suffered other serious Victoza side effects you may be eligible for a Victoza cancer lawsuit. Contact Victoza cancer lawyer Timothy L. Miles today and see if you qualify for a Victoza cancer lawsuit and could be eligible for substantial compensation. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today. TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A MERIDIA LAWSUIT, KEEP ALL YOUR MERIDIA DOCUMENTATION4/24/2023
 If you took Merida as directed and suffered Merida side effects, contact Merida lawyer Timothy L. Miles If you took Merida as directed and suffered Merida side effects, contact Merida lawyer Timothy L. Miles Meridia, made by Abbott Laboratories, was approved in 1997 for weight loss and weight loss maintenance for obese patients, and also very obese individuals who already had other health risk factors for cardiovascular disease. Meridia was approved after clinical studies showed that more patients taking Meridia lost at least 5 percent of their body weight than members of the placebo group who relied solely on diet and exercise. However, the U.S. Food & Drug Administration (FDA) has now requested that Meridia be recalled following a nearly year-long safety review of the drug which was initiated after researchers published the results of a study known as SCOUT (Sibutramine Cardiovascular Outcomes Trial). The study, which prompted a Meridia recall in Europe, showed a 16% percent increase in the risk of serious Meridia side effects, including:
SCOUT monitored 10,000 obese patients over the age of 55 who had a history of heart disease, and type 2 diabetes or similar risk factors. The initial purpose of the study was to show that weight loss through Meridia use lowered the risk of heart problems. However, that was not the case. In fact, the results suggested that those taking the weight loss drug had an increased chance of suffering Meridia cardiovascular side effects. Doctors have been advised to stop prescribing Meridia and begin discussing weight loss alternatives with their patients. If you or a loved one suffered serious Meridia side effects including a heart attack, stroke or even death, contact Meridia lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial compensation. If you or a loved one took Meridia and suffered Meridia side effects and believe are you are eligible for a Merida lawsuit, it is vital that you maintain all you Meridia documentation and other evidence in your possession. You will need this evidence to prove your case and that you are entitled to compensation in a Merida lawsuit as a result of suffering serious Meridia side effects. Read on to learn more about the documentation and other evidence you should maintain if you suffered serious Merida side effects and believe you are you are eligible for a Merida lawsuit. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A MERIDIA LAWSUIT, KEEP ALL YOUR MERIDIA DOCUMENTATIONMaintaining all your Meridia documentation and other evidence is critical if you believe you are eligible for a Meridia lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Meridia lawsuit. Your Meridia lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Meridia documents if you believe you are eligible for a Meridia lawsuit, including:
IF YOU SUFFERED MERIDIA SIDE EFFECTS, CALL A MERIDIA LAWYER TODAY TO SEE IF YOU ARE ELIGIBLE FOR A MERIDIA LAWSUITIf you or a loved one suffered serious Meridia side effects including a heart attack, stroke or even death, contact Meridia lawyer Timothy L. Miles today to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial compensation. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today and see if you meet the requirements for a Meridia lawsuit. TIMOTHY L. MILES, ESQ. Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT, KEEP ALL YOUR TASIGNA DOCUMENTATION4/24/2023
 If you suffered Tasigna side effects, contact a Tasigna lawyer today for a free case evaluation If you suffered Tasigna side effects, contact a Tasigna lawyer today for a free case evaluation First approved in 2007, Tasigna (nilotinib), is a prescription medication used to treat chronic myeloid leukaemia (CML). Tasigna is part of a class of drugs known as tyrosine kinase inhibitors (TKIs). Other TKI drugs include Gleevec (imatinib), also made by Novartis, and Sprycel (dasatinib), made by Bristol-Myers Squibb. These drugs help treat leukemia by blocking the protein tyrosine kinases—a protein that stimulates the growth of cancer cells. In blocking this protein, TKI drugs like Tasigna help stop leukemia from spreading. Tasigna is prescribed as a twice daily drug and is taken orally in capsule form. Injured patients and their families are now filing lawsuits against Tasigna manufacturer Novartis, on the grounds that the company failed to warn of the drug’s cardiovascular risks. Studies have allegedly linked the drug to serious cardiovascular side effects like Long QT syndrome, a condition involving irregular heartbeats, and atherosclerosis, a disease that causes plaque to build up in the arteries. The FDA issued a black box warning (the highest warning they can issue) for Tasigna’s link to QT interval prolongation and sudden death. If you suffered Tasigna side effects, including Tasigna cardiovascular side effects, call Tasigna lawyer Timothy L. Miles today for a free case evaluation and see if you qualify for a Tasigna lawsuit. If you or a loved one took Tasigna and suffered Tasigna side effects and believe are you are eligible for a Tasigna lawsuit, it is vital that you maintain all you Tasigna documentation and other evidence in your possession. You will need this evidence to prove your case and that you are entitled to compensation in a Tasigna lawsuit as a result of suffering serious Tasigna side effects. Read on to learn more about the documentation and other evidence you should maintain if you suffered serious Tasigna side effects and believe you are you are eligible for a Tasigna lawsuit. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT, KEEP ALL YOUR TASIGNA DOCUMENTATIONMaintaining all your Tasigna documentation and other evidence is critical if you believe you are eligible for a Tasigna lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Tasigna lawsuit. Your Tasigna lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Tasigna documents if you believe you are eligible for a Tasigna lawsuit, including:
CALL A TASIGNA LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT?If you or a loved one took Tasigna and suffered heart or circulation problems (including complications like blocked arteries, heart attacks, or strokes), you could be eligible for a Tasigna Lawsuit. If you suffered Tasigna side effects, including Tasigna cardiovascular side effects, call Tasigna lawyer Timothy L. Miles today for a free case evaluation and see if you qualify for a Tasigna lawsuit. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.
If you took Byetta and suffered serious Byetta side effects, contact Byetta lawyer Timothy L. Miles today
Byetta (exenatide) was the first of a new class drugs used to treat type 2 diabetes drugs known as glucagon-like peptide 1 (GLP-1) receptor agonists which generated a lot of early excitement with added benefits including a low risk of hypoglycemia and successful weight loss. However, early optimism was halted by reports that Byetta and similar drugs were a possible risk factor for pancreatitis and pancreatic cancer.
There have been hundreds of pancreatic cancer lawsuits filed against the manufacturers of Byetta, Victoza, Janument, and Januvia. These lawsuits were all transferred the Southern District of California by the Judicial Panel on Multidistrict Litigation for coordinated and pretrial discovery and proceedings. A ruling by the trial judge in 2015 appeared to end the case until a recent court of appeals decision in favor of the Byetta plaintiffs holding their claims may proceed in federal court. If you or a loved one took Byetta and were diagnosed with pancreatic cancer or other serious Byetta side effects, and believe you are you are eligible for a Byetta lawsuit, it is vital that you maintain all you Byetta documentation and other evidence in your possession. You will need this evidence to prove your case and that you are entitled to compensation in a Byetta lawsuit as a result of suffering serious Byetta side effects. Read on to learn more about the documentation and other evidence you should maintain if you suffered serious Byetta side effects and believe you are you are eligible for a Byetta lawsuit. IF YOU BELIEVE YOU ARE ELIGIBLE FOR A BYETTA LAWSUIT, KEEP ALL YOUR BYETTA DOCUMENTATION Call a Byetta lawyer today if you suffered serious Byetta side effects Call a Byetta lawyer today if you suffered serious Byetta side effects
Maintaining all your Byetta documentation and other evidence is critical if you believe you are eligible for a Byetta lawsuit. You will need this evidence to prove your case and that you are entitled to compensation in a Byetta lawsuit. Your Byetta lawyer will determine what evidence best supports your claim, but you should maintain and start gathering all your Byetta documents if you believe you are eligible for a Byetta lawsuit, including:
CALL A BYETTA LAWyer if you believe you are eligible for a Byetta lawsuit
If you or a loved one took Byetta and were diagnosed with pancreatic cancer or suffered or other serious Byetta side effects, you may be eligible to file a Byetta lawsuit. Contact Byetta lawyer Timothy L. Miles today and see if you meet the requirements for a Byetta lawsuit and could be eligible for substantial compensation. (855) Tim-M-Law (855-846-6529).
TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  If you were injured by a defective product or a dangerous drug, call Timothy L. Miles today If you were injured by a defective product or a dangerous drug, call Timothy L. Miles today When a consumer is injured by a defective product, the injured party can seek financial compensation through a product liability lawsuit. However, product liability cases can be very complex and involve many factors -- which is why it is vital to understand your options as soon as you realize you have been hurt by someone else’s negligence. Whether you were hurt by a dangerous drug, unsafe toy, a dangerous food additive, or another type of product defect, understanding your legal rights and responsibilities can help you take action quickly and effectively. This article answers some frequently questions about product liability cases so that you are prepared if an unfortunate event arises. What Is a Product Liability Case?A products liability claim is a cause of action brought by a consumer who was injured by a product that failed to function in a reasonably safe manner. Product liability cases cover a wide range of defective product types and scenarios, but they all have one thing in common: If a manufacturer, supplier, distributor, retailer, or other party failed to follow industry standards for producing or selling a product, and that product caused an injury, that party could be held liable for damages. There are three types of product liability claims: Manufacturing defects, design defects, and marketing defects. WHAT DOES A PRODUCTS LIABILITY LAWYER DO?A products liability lawyer represents individuals who have been harmed by defective consumer goods including medical devices, dangerous drugs, toys, automobiles, home appliances, food, toxic chemicals and other goods people buy and use every day. Who Can Be Held Liable for a Defective Product?In order to hold a manufacturer or seller liable for a defective product, you must be able to demonstrate that the product was defective when it left the defendant's possession. In a product liability case, the injured party can sue one or more parties involved in the creation and distribution of the product. These parties include: Product designers/engineers, product manufacturers, product inspectors/quality assurance testers, and product suppliers. Product distributors, retailers, and other parties who are not part of the production process may also be liable for damages in certain cases. An experienced product liability lawyer like Timothy L. Miles can help you determine which parties are most responsible for the defects in your specific case. What Constitutes a Defective Product?A product is considered defective when it does not meet the reasonable standard of safety that a reasonable person would expect under the circumstances. Of course, this can vary depending on the type of product involved. For example, foods and medication have different reasonable safety standards than household appliances. A defective product can create a number of risks for an end user. In some cases, the design of the product itself is the issue -- such as a table whose legs are too short to support its weight. When Can You Pursue a Product Liability Claim?You should report your injuries to the appropriate government agency as soon as you realize you have been hurt by a defective product. Failure to report the defect could jeopardize your legal rights, as it is important to identify the underlying cause of your injuries and file the appropriate claim as quickly as possible. You should also contact a top-rated lawyer who is experienced in product liability cases such as Nashville attorney Timothy L. Miles. Product liability cases can be complicated and depend on a wide range of factors, such as the specific nature of the product defect, the extent of your injuries, and the jurisdiction of your case. The sooner you begin working with an experienced in attorney, the more likely you are to find success in your case. What Damages Can I Recover If I Prevail in A Defective Product Case?If a plaintiff proves the product was defective, they are entitled to recover damages for: past and future wage loss; past, current and future medical treatment; damages for pain, suffering and emotional distress; and, if the plaintiff can meet the standard, punitive damages (i.e., damages intended to punish the defendant and deter future similar conduct). How Do I Establish Liability in a Defective Product Case?You must prove the product was defective and that the defect caused your injuries and damages. You must show that you would not have been injured if the product had not been defective, and you must have been using the product in an intended or reasonably foreseeable way at the time. What Is a Strict Liability Claim?This theory of liability makes the manufacture and seller of the product responsible for all defective products that unreasonably threaten the personal safety of a consumer or the consumer’s property, regardless of who is at fault. WHAT IS NEGLIGENCE?Negligence means that a manufacturer may be liable for a defective consumer product if it was foreseeable that a product defect would cause injury but did not act in a reasonable manner to prevent it. WHAT IS A WARRANTY?An inherent promise that comes with a consumer product that ensures that the product is fit for its intended use. WHAT IS A FAILURE TO WARN PRODUCTS LIABILITY CLAIM? If you suffered side effects from a drug that failed to ward of the side effects, call dangerous drug lawyer Timothy L. Miles If you suffered side effects from a drug that failed to ward of the side effects, call dangerous drug lawyer Timothy L. Miles In other cases, the issue is with the instructions or warnings given with the product -- such as a failure to warn claim that plaintiffs are asserting in the various dangerous drug cases. A failure to warn claim asserts the defendant manufacturer did not supply adequate warnings or instructions on how to use the product safely, and the plaintiff was injured due to the failure to disclose the risk. Warning labels may not always be visible on a product. The manufacturer might put a warning on the box or in the instructions. A warning may not be sufficient to protect a manufacturer from liability if the user will not see it. For example, a manufacturer might have to put a warning directly on a product if people who would face the risk will not see it on the box or in the instructions. In addition, warnings should be expressed in language that the average individual understands, even if the product is complex and technical. For example, plaintiffs in the Elmiron lawsuit allege that its manufacturer Janssen Pharmaceuticals failed to warn consumers that the drug may cause a serious eye condition in those taking it, one that may result in blindness. Similarly, the Belviq lawsuit alleges the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. In both the Kombiglyze XR lawsuit and the Onglyza lawsuit plaintiffs allege that the defendants failed to warn or mitigate the risk of cardiovascular injuries despite their knowledge. The Xeljanz lawsuits claim that Pfizer failed to give adequate warnings about the Xeljanz side effects, which caused preventable harm to patients who suffered blood clots in the lungs, heart attacks, strokes, breast and colon cancer, and other severe health problems. Both the Valsartan lawsuit and Tasigna lawsuit likewise allege the defendants made a defective drug and failed to warn physicians about serious Valsartan side effects and Tasigna side effects including their carcinogenic properties. Hundreds of plaintiffs have filed a Victoza cancer lawsuit and allege that Victoza, which is an incretin mimetic drug used to treat type 2 diabetes, may cause pancreatic cancer and that the manufacturer of Victoza, Novo Nordisk, failed to warn them of this risk and other Victoza side effects. Similarly, plaintiffs who took Byetta, another medicine used to treat type 2 diabetes, have filed a Byetta lawsuit alleging it too may cause pancreatic cancer and other Byetta side effects and that the manufacturers failed to warn doctors and patients of the risks. The Actemra lawsuit alleges the defendant failed to warn of serious Actemra side effects including heart, lung, or pancreatic side effects. Patients who took Meridia and subsequently developed Meridia Side Effects including a heart attack, stroke or even death allege in a Meridia lawsuit the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. Finally, many plaintiffs who took Tepezza have filed a Tepezza lawsuit and allege the labels on the drug did not warn patients or doctors about serious Tepezza side effects, most notably, the potential risk for Tepezza hearing loss nor did it warn how these issues could be permanent to those taking the eye drug. In yet other cases, the product's packaging may be the cause -- such as a toy that contains lead paint. What Are the Common Elements of a Products Liability Claim?Regardless of the legal theory that the lawsuit is brought pursuant to, there are three common elements to all product liability lawsuits, The lawsuit must involve a product, the product must be found to be defective, and the product defect must be found to be the proximate cause of the injury. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  If you suffered side effects from a drug that failed to warn of the side effects call dangerous drug lawyer Timothy L. Miles If you suffered side effects from a drug that failed to warn of the side effects call dangerous drug lawyer Timothy L. Miles The majority of product liability claims are brought due to a product suffering from some type of manufacturing or design defect. In many cases, there has been a voluntary recall or a recall ordered by the U.S. Food & Drug Administration (FDA). The types of defective product cases typically assert a design or manufacturing defect and include, among others: defective medical devices; automobile defects; dangerous or defective pharmaceutical drugs, and toxic or environmental torts. For example, plaintiffs in the Elmiron lawsuit allege that its manufacturer Janssen Pharmaceuticals failed to warn consumers that the drug may cause a serious eye condition in those taking it, one that may result in blindness. Similarly, the Belviq lawsuit alleges the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. In both the Kombiglyze XR lawsuit and the Onglyza lawsuit plaintiffs allege that the defendants failed to warn or mitigate the risk of cardiovascular injuries despite their knowledge. The Xeljanz lawsuits claim that Pfizer failed to give adequate warnings about the Xeljanz side effects, which caused preventable harm to patients who suffered blood clots in the lungs, heart attacks, strokes, breast and colon cancer, and other severe health problems. Both the Valsartan lawsuit and Tasigna lawsuit likewise allege the defendants made a defective drug and failed to warn physicians about serious Valsartan side effects and Tasigna side effects including their carcinogenic properties. Hundreds of plaintiffs have filed a Victoza cancer lawsuit and allege that Victoza, which is an incretin mimetic drug used to treat type 2 diabetes, may cause pancreatic cancer and that the manufacturer of Victoza, Novo Nordisk, failed to warn them of this risk and other Victoza side effects. Similarly, plaintiffs who took Byetta, another medicine used to treat type 2 diabetes, have filed a Byetta lawsuit alleging it too may cause pancreatic cancer and other Byetta side effects and that the manufacturers failed to warn doctors and patients of the risks. The Actemra lawsuit alleges the defendant failed to warn of serious Actemra side effects including heart, lung, or pancreatic side effects. Patients who took Meridia and subsequently developed Meridia Side Effects including a heart attack, stroke or even death allege in a Meridia lawsuit the defendants made a defective drug and failed to warn physicians about its carcinogenic properties. Finally, many plaintiffs who took Tepezza have filed a Tepezza lawsuit and allege the labels on the drug did not warn patients or doctors about serious Tepezza side effects, most notably, the potential risk for Tepezza hearing loss nor did it warn how these issues could be permanent to those taking the eye drug. What Is a Failure to Warn Products Liability Claim? If your medication failed to warn of unwanted side effects call dangerous drugs lawyer Timothy L. Miles If your medication failed to warn of unwanted side effects call dangerous drugs lawyer Timothy L. Miles A failure to warn claim asserts the defendant manufacturer did not supply adequate warnings or instructions on how to use the product safely, and the plaintiff was injured due to the failure to disclose the risk. Warning labels may not always be visible on a product. The manufacturer might put a warning on the box or in the instructions. A warning may not be sufficient to protect a manufacturer from liability if the user will not see it. For example, a manufacturer might have to put a warning directly on a product if people who would face the risk will not see it on the box or in the instructions. In addition, warnings should be expressed in language that the average individual understands, even if the product is complex and technical. Defenses to a Failure to Warn ClaimThe Danger Was EvidentOne defense to a failure to warn claim is that the danger was evident. While the law may vary in jurisdictions, one common rule is that an obvious risk does not require a warning. However, if an ordinary person using common sense would not be able to recognize the risk, the defense would not be available. For example, the manufacturer of toothpaste that caused headaches, could not rely on this defense since an ordinary person would not expect toothpaste to cause headaches. The Misuse Was Not ForeseeableA defendant manufacturer may also argue the plaintiff did not use the product in a reasonably foreseeable way. If someone uses a product in an unpredictable manner, then manufacturer will not be liable for a failure to warn. For example, if someone buys a bicycle, but instead of ridding it, turns it up on its handlebars and tries to juggle while balancing on the wheels, a manufacturer will not be liable because it did not warn about a risk that it obviously could not have reasonably foreseen. However, a plaintiff does not have to use a product exactly as it was intended by the manufacturer, and a manufacturer’s lack of warning for a predictable misuse may still be liable if the risk of was not obvious. This obviously will not be at issue in the baby formula recall cases since the product was being used exactly as intended. Manufacturer KnowledgeA manufacturer may try to avoid liability by arguing it had no knowledge about the risk it failed to provide warnings about. But, to state a claim for relief, actual knowledge is not required. Instead, the test is whether the manufacturer reasonably should have known about the risk. If the answer is yes, then the manufacturer may be held liable. In other words, a defendant may be liable for a risk it should have known about, even if the defendant possessed no actual knowledge of the risk. ConclusionIf you suffered serious side effects from a medication that failed to warn of the side effects, contact dangerous drug lawyer Timothy L. Miles today for a free case evaluation. You could be eligible to file a lawsuit alleging a failure to warn and could potentially be entitled to substantial compensation. timothy l. miles, esq.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  If you took Merida as directed as developed Merida side effects, call a Merida lawyer today If you took Merida as directed as developed Merida side effects, call a Merida lawyer today Meridia, made by Abbott Laboratories, was approved in 1997 for weight loss and weight loss maintenance for obese patients, and also very obese individuals who already had other health risk factors for cardiovascular disease. Meridia was approved after clinical studies showed that more patients taking Meridia lost at least 5 percent of their body weight than members of the placebo group who relied solely on diet and exercise. However, the U.S. Food & Drug Administration (FDA) has now requested that Meridia be recalled following a nearly year-long safety review of the drug which was initiated after researchers published the results of a study known as SCOUT (Sibutramine Cardiovascular Outcomes Trial). The study, which prompted a Meridia recall in Europe, showed a 16% percent increase in the risk of serious Meridia side effects, including:
SCOUT monitored 10,000 obese patients over the age of 55 who had a history of heart disease, and type 2 diabetes or similar risk factors. The initial purpose of the study was to show that weight loss through Meridia use lowered the risk of heart problems. However, that was not the case. In fact, the results suggested that those taking the weight loss drug had an increased chance of suffering Meridia cardiovascular side effects. Doctors have been advised to stop prescribing Meridia and begin discussing weight loss alternatives with their patients. WHAT IS MERIDIA? Call a Merida lawyer if you suffered severe Merida side effects Call a Merida lawyer if you suffered severe Merida side effects Meridia is a medication which requires a prescription and is used to treat the symptoms of obesity, weight loss, and maintenance of weight loss. It may be used alone or with other medications. Meridia belongs to a class of drugs called Schedule IV controlled substances and can cause severe Meridia side effects in some people. If you or a loved one suffered severe Meridia side effects call Meridia Lawyer Timothy L. Miles for a free case evaluation to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial damages. SERIOUS MERIDIA SIDE EFFECTSIn the beginning, Meridia seemed to be a booming success as it was used by millions of individuals around the world. However, hundreds of users began reporting serious Meridia side effects including:
Patients taking Meridia have also experienced:
Additionally, approximately 30 deaths were caused by Merida in the United States. Researchers looked at 10,000 patients who were obese, over the age of 55 with a history of risk factors and found that 11.4% of patients using Meridia suffered heart problems, compared to 10% given a sugar pill. As of June 30, 2009, the FDA Adverse Event Reactions (AERS) database indicates that at least 84 Meridia deaths from cardiovascular causes have been reported to the FDA. If you suffered severe Meridia side effects call Meridia Lawyer Timothy L. Miles for a free case evaluation to see if you are eligible for a Meridia lawsuit. MERIDIA RECALLED AFTER SERIOUS MERIDIA SIDE EFFECTS REPORTEDThe limited benefits provided by Meridia are outweighed by the increased risk of cardiovascular problems which prompted federal regulators to issue the recall for Meridia in October 2010. Lawyers are investigating and considering potential lawsuits against Abbott on behalf of individuals who suffered a:
If you took Meridia as directed and suffered serious Meridia side effects including a heart attack or stroke, then you may meet the requirements for a Merida lawsuit and possibly be entitled to substantial compensation. Contact Merida Lawyer Timothy L. Miles today to see if you are eligible for a Merida lawsuit. SHOULD I REPORT MERIDIA SIDE EFFECTS?Yes, the FDA urges consumers who experience adverse advents to any medication or product to report it via the MedWatch Online Voluntary Reporting Form. When reporting your Merida side effects make sure to be through and give as many details as possible about your experience taking the drug including all Merida side effects you suffered, and any other symptoms you suffered that you believe was caused by Merida use. IF YOU SUFFERED MERIDIA SIDE EFFECTS, CALL A MERIDIA LAWYER TODAYIf you or a loved one suffered serious Meridia side effects including a heart attack, stroke or even death, contact Meridia lawyer Timothy L. Miles today to see if you are eligible for a Meridia lawsuit and possibly entitled to substantial compensation. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today and see if you meet the requirements for a Meridia lawsuit. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.
If you or a loved one took Byetta and were diagnosed with pancreatic cancer, Contact Byetta lawsuit lawyer Timothy L. Miles today
Byetta (exenatide) was the first of a new class drugs used to treat type 2 diabetes drugs known as glucagon-like peptide 1 (GLP-1) receptor agonists which generated a lot of early excitement with added benefits including a low risk of hypoglycemia and successful weight loss. However, early optimism was halted by reports that Byetta and similar drugs were a possible risk factor for pancreatitis and pancreatic cancer.
There have been hundreds of pancreatic cancer lawsuits filed against the manufacturers of Byetta, Victoza, Janument, and Januvia. These lawsuits were all transferred the Southern District of California by the Judicial Panel on Multidistrict Litigation for coordinated and pretrial discovery and proceedings. A ruling by the trial judge in 2015 appeared to end the case until a recent court of appeals decision in favor of the Byetta plaintiffs holding their claims may proceed in federal court. If you or a loved one took Byetta and were diagnosed with pancreatic cancer, contact Byetta lawsuit lawyer Timothy L. Miles today to see if you are eligible for Byetta lawsuit and possible entitled to substantial damages. (855) Tim-M-Law (855-846-6529). what is byetta?
Byetta (exenatide) is a medicine used to treat type 2 diabetes. It is glucagon-like peptide-1 (GLP-1) receptor agonist. A receptor agonist drug means that it binds to a certain receptor and causes the same action as the substance that normally binds to that receptor. Byetta, by binding to and activating the GLP-1 receptor, tells your body to release more insulin, stops the body from releasing more sugar, and slows down digestion.
Byetta is a solution for injection available in pre‑filled pens of 5mcg or 10 mcg. The shots are given by the patient twice a day by injection under the skin in the abdomen, thigh or upper arm. The starting dose of Byetta is 5 mcg, twice a day before a meal. After four weeks, the dose can be increased to 10 mcg depending on how well your blood sugar levels respond to Byetta injections. WHAT IS THE PROBLEM WITH BYETTA?
Incretin mimetic drugs such as Byetta regulate the production of incretin, a type of hormone that stimulates the release of insulin in the body. Byetta does this by injecting patients with a substance that mimics a particular incretin called Glucagon-Like Peptide-1 (GLP-1). Byetta lawsuits allege this process overstimulates the pancreas causing cells in the pancreas to multiply resulting in pancreatic cancer.
BYETTA SIDE EFFECTS
Byetta side effects include, among others:
A more severe allergic reaction to Byetta is rare but possible. The symptoms of a severe allergic reaction can include:
IS THERE A LINK BETWEEN BYETTA AND PANCREATIC CANCER?
A number of scientific studies support plaintiff claims that Byetta causes pancreatic cancer.
WHAT ARE THE SYMPTOMS OF PANCREATITIS?
Pancreatitis, an inflammation of the pancreas, can cause the following symptoms, among others:
It is not known for certain if Byetta or other similar incretin mimetic diabetes drugs can cause pancreatic cancer. However, Incretin mimetics treat type 2 diabetes by mimicking the action of GLP-1, a hormone that stimulates the pancreas to produce more insulin. In certain people, this could possibly cause chronic inflammation of the pancreas, also known as pancreatitis, which is a known risk factor for pancreatic cancer. CALL A BYETTA LAWSUIT LAWYER IF YOU TOOK BYETTA AND WERE DIAGNOSED WITH PANCREATIC CANCER
If you or a loved one took Byetta and were diagnosed with pancreatic cancer, you may be eligible to file a Byetta lawsuit. Contact Byetta lawsuit lawyer Timothy L. Miles today and see if you meet the requirements for a Byetta lawsuit and could be eligible for substantial compensation. (855) Tim-M-Law (855-846-6529).
TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.
If you took Xeljanz and developed blood clots, contact Xelzanz Lawyer Timothy L. Miles today to see if you are eligible for a Xeljanz lawsuit
Pharmaceutical companies and suppliers have both an ethical and legal responsibility to properly disclose the risks and side effects of a drug before bringing it to market, but all too often those warnings are withheld in the name of profit.
However, in just the last two years, the U.S. Food and Drug Administration (FDA) has warned that Xeljanz (Tofacitinib) can be associated with heart problems, blood clots, and even cancer. But the question remains: why didn’t Pfizer warn consumers about these potential risks sooner? Xeljanz lawsuits claim that Pfizer failed to give adequate warning about the Xeljanz side effects, which caused preventable harm to patients who took Xeljanz and developed blood clots in the lungs, heart attacks, strokes, breast and colon cancer, and other severe health problems. We are accepting these cases on a contingency-fee basis. That means our clients don’t pay unless we win. If you need to discuss a lawsuit with a Xeljanz lawyer, free of charge, please contact us. (855) Tim-M-Law (855-846-6529). Read on to learn more about the Xeljanz side effects. what is XELJANZ If you or a loved one took Xeljanx and developed Xeljanx side effects call a Xeljanz law today If you or a loved one took Xeljanx and developed Xeljanx side effects call a Xeljanz law today
Xeljanz belongs to a class of drugs known as Janus kinase (JAK) inhibitors. As Pfizer explains, our body has signaling proteins called cytokines that regulate immune system responses. The production of cytokines is increased when our body fights infection. Cytokine production is thought to play a role in chronic inflammatory conditions, such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. Xeljanz disrupts signaling that produces some of the cytokines, reducing inflammation. Specifically, Xeljanz blocks—or inhibits—the release of cytokines in the JAK signal pathway.
Xeljanz has been approved for several different uses:
Xeljanz has been approved for several different uses:
WHAT ARE THE XELJANZ SIDE EFFECTS?
The Xeljanz Side Effects include:
POST-MARKETING XELJANZ STUDY REVEALS SERIOUS SAFETY CONCERNS
Most Americans assume that if a drug is approved by the FDA, it has been thoroughly evaluated for safety and efficacy. However, this is not always the case. In addition to pre-approval clinical testing, the FDA may order post-marketing studies as a condition of drug approval. These trials gather more information about a drug’s safety, efficacy, or optimal use. Here, the Xeljanz study reveals serious safety concerns about the Xeljanx side effects.
When the FDA first approved Xeljanz in 2012, it ordered a post-marketing Xeljanz safety study known as ORAL Surveillance. But rather than confirming the safety of Xeljanz, the post-marketing Xeljanz Study Revealed Serious Safety Concerns and has led to new health alerts and warnings. SOME PATIENTS TOOK XELJANZ AND DEVELOPED BLOOD CLOTS
In February 2019, the FDA warned that the ongoing Xeljanz safety trial found an increased risk of taking Xeljanz and developing blood clots in the lungs and death. In July 2019, the FDA announced that it had approved new warnings about thrombosis and death in patients treated with a 10 mg twice-daily dose of Xeljanz.
Thrombosis occurs when a blood clot blocks a vein or artery. Deep vein thrombosis, pulmonary embolism, and arterial thrombosis have all been reported in Xeljanz patients. A blood clot in a deep vein is known as a deep vein thrombosis (DVT). DVT usually occurs in the legs. A blood clot that travels to the lungs is called a pulmonary embolism (PE). PE is usually caused by a blood clot that starts in the arm or the leg. Arterial thrombosis is a blood clot in an artery. This type of thrombosis can stop blood from reaching vital organs. Blood clots can lead to a heart attack, stroke, organ damage, and even death. Symptoms of a blood clot include:
XELJANZ AND CANCER
The next major safety news to come out of the Xeljanz post-marketing study was equally concerning because of Xeljanz and Cancer. The FDA alerted the public in February 2021 that preliminary results showed an increased risk of cancer with Xeljanz.
Pfizer reports in a press release that malignancies observed in Xeljanz studies include (but are not limited to):
Also in February 2021, the FDA warned about serious heart-related problems that may result from Xeljanz use. Pfizer refers to these in its press release as “major adverse cardiovascular events” (MACE). Pfizer reports that a total of 135 patients in the study had a major adverse cardiovascular event, although the full results have not yet been released. The most frequently reported event was myocardial infarction (i.e. heart attack).
Patients who took Xeljanz as prescribed and developed a malignancy, thrombosis, or heart-related issues may qualify for a lawsuit. Lawsuits against Pfizer can recover compensation for medical bills, lost wages, pain and suffering, reduced quality of life, and more. XELJANZ AND MAJOR CARDIOVASCULAR EVENTS
The latest results from the ORAL Surveillance study were released in early 2021 and led to an FDA safety communication about increased risk of serious heart-related problems, or “major adverse cardiovascular events” (MACE).
Pfizer reported 135 patient subjects with MACE. The most frequently reported such event was myocardial infarction (heart attack), but the full data has not yet been released. Other major cardiac events that may be linked to Xeljanz include:
SHOULD I REPORT XELJANZ SIDE EFFECTS?
Yes, the FDA is encouraging people who had a problem related to the recalled devices or side effect to drugs to report the problem through the MedWatch Voluntary Reporting Form or call 1-800-332-1088 for more information. Make sure to provide all information about your Xeljanz Side Effects including with a detailed description of any problems you suffered while taking Xeljanz.
CONTACT A XELJANZ LAWYER ABOUT A XELJANZ LAWSUIT
Xeljanz, while marketed as a safe medication, increases the risk of blood clots, cancer, and cardiovascular problems for its users. The FDA approved the drug upon release, but a post-marketing safety study revealed serious safety concerns and concluded that the side effects are more detrimental than initially believed. The company responsible for the medication, Pfizer, knew about the side effects prior to the safety study but failed to disclose this information to the public in an attempt to retain their profits. This unethical behavior exposed thousands of consumers to an elevated risk of harm, making their actions unacceptable by any means.
Unfortunately, some users of Xeljanz have developed cancer as a result of their medication use, including but not limited to lymphoma, melanoma, and lung cancer. Pfizer, already knowing the risk of the Xeljanz side effects, sold it to consumers anyway, causing many innocent individuals to develop and suffer from a life-threatening disease including taking Xeljanx and developing blood clots. Their reluctance to disclose the risk of their product is not just unethical, it is illegal, and our attorneys are here to help hold them accountable to the full extent of the law. Contact a Xeljanz Lawyer today at (855) 846-6529 or [email protected] or by submitting the form here and see if you meet the Xeljanz lawsuit criteria if you suffered Xeljanz side effects. Someone will promptly get in touch with you. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.
If you or a loved one suffered serious Actemra side effects while taking Actemra, contact Actemra lawyer Timothy L Miles today
Roche’s Actemra was heralded as a breakthrough therapy for rheumatoid arthritis when it was introduced in 2010.
The drug’s website proclaims that “Actemra works differently” and shows a patient happily scuba diving and walking on a tropical beach. But nowhere does Roche mention certain side effects that may have contributed to more than 1,100 patient deaths. Research suggests that Actemra patients, compared to those taking competing rheumatoid arthritis drugs, face similar risks for heart attacks, heart failure, strokes, lung disease, and pancreatitis. But unlike the competition, Actemra’s label does not warn about these side effects. If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L. Miles today to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation. Read on for answers to the three most frequently asked questions about an Actemra lawsuit. WHAT ARE THE ACTEMRA SIDE EFFECTS TO THE HEART AND LUNGS? Call Actemra lawyer today if you suffered serious Actemra side effects Call Actemra lawyer today if you suffered serious Actemra side effects
A STAT News report and postmarketing studies raise questions about the adequacy of Actemra's safety warnings.
STAT reviewed more than 500,000 adverse event reports on leading rheumatoid arthritis drugs, including Actemra, Humira, and Remicade. STAT found evidence that Roche downplayed Actemra’s cardiovascular, pancreatic, and lung side effects. According to STAT, from 2010 to 2016 the U.S. Food and Drug Administration (FDA) logged more than 13,500 Actemra adverse event reports, including 1,128 patients who died after taking Actemra. While safety data indicate the risks of heart attacks, strokes, heart failure, and interstitial lung disease are as high or higher for Actemra patients than for patients taking some similar drugs, Actemra’s warning label—unlike its competition's—does not reflect these risks. For example, Actemra patients reported more cases of interstitial lung disease than Remicade patients, and nearly the same number as Humira patients. But while Remicade and Humira have interstitial lung disease warnings, Actemra does not. STAT’s findings are supported by postmarketing studies—clinical studies conducted after a pharmaceutical product goes on the market to better understand drug risks. The FDA ordered Actemra postmarketing studies because premarket testing revealed potentially worrisome side effects. One such study compared Actemra to another arthritis drug, Enbrel, and found rates of stroke and heart failure were about 1.5 times higher in Actemra users. Enbrel has strong warnings for patients with cardiovascular disease. Actemra does not have similar warnings despite similar or worse cardiovascular risks. The takeaway from these findings is that doctors and patients may not be getting a complete picture of Actemra’s risk-benefit profile. HOW DO I KNOW IF I AM ELIGIBLE FOR AN ACTEMRA LAWSUIT?
We are looking into claims that Genetech/Roche failed to warn about the following Actemra side effects:
Actemra patients who suffered any of these Actemra side effects may be eligible for an Actemra lawsuit. IF I MEET THE REQUIREMENTS FOR AN ACTEMRA LAWSUIT, WHAT CAN I GET?
If you meet the requirements for an Actemra lawsuit and are successful you can recover money for some of the following damages:
• Past and future medical bills (including medication, hospital stays, and in-home care) • Pain and suffering • Lost wages • Loss of earning capacity • Funeral expenses (in the event of a loved one’s death) Broadly speaking, a plaintiff who is eligible for an Actemra lawsuit could be entitled to compensation for any past and future costs associated with their diagnosis if they meet the requirements for an Actemra lawsuit and are successful. CALL AN ACTEMRA LAWYER TODAY IF YOU SUFFERED SERIOUS ACTEMRA SIDE EFFECTS?
If you or a loved one suffered serious Actemra side effects including heart, lung, or pancreatic side effects while taking Actemra, contact Actemra lawyer Timothy L Miles today to see if you are eligible for an Actemra lawsuit. If you meet the requirements for an Actemra lawsuit you may be entitled to substantial compensation.
TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  Call a Tepezza lawyer if you suffered Tepezza hearing loss Call a Tepezza lawyer if you suffered Tepezza hearing loss Tepezza (teprotumumab-trbw) is a prescription drug that was developed by Horizon Therapeutics® for a condition known as Graves' eye disease and the treatment of thyroid eye disease (TED). TED is a condition that occurs when the eye muscles, tear glands, eyelids, and the fatty tissue behind the eyes become inflamed. The eye inflammation is triggered through an abnormal autoimmune response and can cause the eyes to swell and obstruct vision. HOW DOES TEPEZZA WORK?Tepezza works by blocking a specific protein that is believed to be the cause of the development of TED. The eye treatment drug is administered through injection about every three weeks for roughly five months. Approved by the Food and Drug Administration (FDA) in January 2020, it was the first medication approved for TED. WHAT ARE TEPEZZA LAWSUITS ABOUT?Victims of Tepezza are now filing lawsuits after taking the drug and suffering from Tepezza hearing loss. According to the lawsuits, the labels on the drug did not warn patients or doctors about the potential risk for hearing loss nor did it warn how these issues could be permanent to those taking the eye drug. TEPEZZA AND HEARING LOSSIn January 2020, the FDA approved Tepezza, the only drug known to treat TED, based on clinical trials that documented only 10% of patients reporting hearing issues. However, a 2021 study found that patients taking Tepezza are at a 65% higher risk for developing temporary and even permanent hearing issues, making it over six times higher than the original estimated risk of hearing loss disclosed by Horizon during the FDA approval process. WHAT ARE THE TEPEZZA SIDE EFFECTS?The Tepezza side effects include:
HOW DO I KNOW IF I QUALIFY FOR A TEPEZZA LAWSUIT? Call a Tepezza lawyer to see if you qualify for a Tepezza lawsuit Call a Tepezza lawyer to see if you qualify for a Tepezza lawsuit In order to qualify for a Tepezza lawsuit, you must have taken the drug and have experienced permanent or persistent Tepezza hearing loss. The best way to understand if you qualify to file a Tepezza lawsuit against Horizon Therapeutics is by speaking to a Tepezza lawyer as soon as possible. Depending on your state, there may be a statute of limitations for filing a claim, ranging from two to four years on average depending on the State. If you suspect that you may have lost your hearing after taking Tepezza, do not wait to speak with an attorney. IF YOU SUFFERED TEPEZZA HEARING LOSS, CALL A TEPEZZA LAWYER TODAY AND SEE IF YOU ARE ELIGIBLE FOR A TEPEZZA LAWSUITIf you or your loved one received Tepezza (teprotumumab) infusions and later experienced Tepezza hearing loss or tinnitus, contact Tepezza lawyer Timothy L. Miles today for a free case evaluation to see if you qualify for a Tepezza Lawsuit and are possibly entitled to significant compensation. timothy l. miles, esq.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.  If you suffered Tasigna side effects contact a Tasigna lawyer about a free case evaluation If you suffered Tasigna side effects contact a Tasigna lawyer about a free case evaluation First approved in 2007, Tasigna (nilotinib), is a prescription medication used to treat chronic myeloid leukaemia (CML). Tasigna is part of a class of drugs known as tyrosine kinase inhibitors (TKIs). Other TKI drugs include Gleevec (imatinib), also made by Novartis, and Sprycel (dasatinib), made by Bristol-Myers Squibb. These drugs help treat leukemia by blocking the protein tyrosine kinases—a protein that stimulates the growth of cancer cells. In blocking this protein, TKI drugs like Tasigna help stop leukemia from spreading. Tasigna is prescribed as a twice daily drug and is taken orally in capsule form. WHAT IS THE PROBLEM WITH TASIGNA?Injured patients and their families are now filing lawsuits against Tasigna manufacturer Novartis, on the grounds that the company failed to warn of the drug’s cardiovascular risks. Studies have allegedly linked the drug to serious cardiovascular side effects like Long QT syndrome, a condition involving irregular heartbeats, and atherosclerosis, a disease that causes plaque to build up in the arteries. The FDA issued a black box warning (the highest warning they can issue) for Tasigna’s link to QT interval prolongation and sudden death. WHAT ARE THE TASIGNA CARDIOVASCULAR SIDE EFFECTS?To date, approximately 15,500 Tasigna related adverse events and 2,786 deaths have allegedly been reported to the FDA to date. These include serious Tasigna side effects including cardiovascular side effects, like Long QT syndrome, myocardial ischemia, and atherosclerosis. Tasigna has one black box warning—the highest warning the FDA can issue—for Long QT syndrome, or QT interval prolongation, and sudden death. It is a condition that affects heart rhythm, resulting in fast, irregular heartbeats. If left untreated, it can result in fainting, seizures, and sudden death. Tasigna may also been linked to myocardial ischemia, a condition involving reduced blood flow to the heart, causing a decreased amount of oxygen sent to the heart. Myocardial ischemia is usually caused by a blockage of the coronary arteries. Recent lawsuits have also been filed over the drug’s alleged connection to atherosclerosis, a disease that causes fatty deposits to build up and eventually clog arteries. This serious condition gradually reduces blood flow and oxygen to cells. Atherosclerosis is currently the leading cause of death in the developed world. It may eventually cause the following complications:
ARE THERE STUDIES LINKING TASIGNA TO CARDIOVASCULAR EVENTS?Two recent studies have connected Tasigna with cardiovascular events:
The FDA’s adverse event reporting database has received more than 100 reports of Tasigna-related myocardial ischemia diagnoses. Myocardial ischemia is a condition that is usually caused by a blockage of the coronary arteries. It results in reduced blood flow to the heart, decreasing the amount of oxygen to the heart. In an FDA post-market review, 5.8% of patients taking Tasigna allegedly suffered ischemic heart. Atherosclerosis is a disease that causes fatty deposits to build up and eventually clog arteries. This serious condition gradually reduces blood flow and oxygen to cells. Atherosclerosis may eventually cause coronary artery disease, peripheral arterial disease, blood clots, heart attacks, or strokes. It’s currently the leading cause of death in the developed world. Since 2013, Tasigna products sold in Canada come with a warning of the drug’s connection to atherosclerosis. This label change came after Canadian health officials warned that 277 cases of atherosclerosis were allegedly reported worldwide. Lawsuits allege that Novartis never warned of the risk of atherosclerosis in the U.S. though, despite these reports. Other serious Tasigna side effects noticed by the FDA that may be linked to Tasigna include low blood cell counts; decreased blood flow to the heart, lungs, or brain; pancreatitis; and Tumor Lysis Syndrome, a condition that can occur when cancer cells are broken down quickly, resulting in kidney failure or an abnormal heartbeat. Lawsuits have been filed some patients against Novartis. who took Tasigna and subsequently suffered a heart or circulation problem. The lawsuits allege Novartis failed to properly warn of these side effe WHAT ARE SOME OF THE TASIGNA SIDE EFFECTS?Novartis warns of the following Tasigna side effects:
HOW DO I KNOW IF I AM ELIGIBLE FOR A TASIGNA LAWSUIT?If you or a loved one took Tasigna and suffered heart or circulation problems (including complications like blocked arteries, heart attacks, or strokes), you could be eligible for a Tasigna lawsuit and possible entitled to substantial compensation. Call Tasigna lawyer Timothy L. Miles today for a free case evaluation to see if you are eligible for a Tasigna lawsuit. CALL A TASIGNA LAWYER TO SEE IF YOU ARE ELIGIBLE FOR A TASIGNA LAWSUIT?If you or a loved one took Tasigna and suffered heart or circulation problems (including complications like blocked arteries, heart attacks, or strokes), you could be eligible for a Tasigna Lawsuit. If you suffered Tasigna side effects, including Tasigna cardiovascular side effects, call Tasigna lawyer Timothy L. Miles today for a free case evaluation and see if you qualify for a Tasigna lawsuit. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more.
If you or a loved one took Valsartan and later suffered serious Valsartan Side Effects, contact Valsartan lawyer Timothy L. Miles today
Patients who have taken Valsartan and have been diagnosed with cancer, as a result, should read on to learn more about how they can hold the drug makers accountable.
Valsartan has been recalled due to possible nitrosamine contamination. Nitrosamines can cause tumors in the liver and other organs in lab animals and are thought to be carcinogenic in humans as well. It is believed that these blood pressure drugs were tainted during flawed manufacturing processes. The U.S. Food and Drug Administration (FDA) stated that these contaminants “are of special concern to global regulators because, unlike most impurities in drugs, they have the potential to cause harm at very low levels.” The recalls began in July when the FDA found that some Valsartan products contained a potentially cancer-causing chemical. European regulators had already made the same discovery. The substance can form during manufacturing if the chemical reactions used to make the drug are not carefully controlled and monitored, the FDA said. Valsartan is made by Chinese manufacturer Zhejiang Huahai Pharmaceuticals. These recalls are a product of greater FDA scrutiny of foreign drug imports, but FDA warnings alone will not ensure safe medications. In a culture where the profit motive exists, drug companies must be held accountable to cutting corners and sacrificing consumer safety. If you or a loved one took Valsartan and later were diagnosed with cancer or other serious Valsartan Side Effects, contact Valsartan lawyer Timothy L. Miles today to see if you are eligible for a Valsartan lawsuit and possibly entitled to substantial compensation. HAVE LAWSUITS BEEN FILED OVER POSSIBLE VALSARTAN SIDE EFFECTS?
Yes, patients who filed Valsartan Cancer Lawsuits were prescribed Valsartan claim that drug manufacturers willfully disregarded required safety procedures at the plant where contamination occurred. The contaminant was undiscovered for years until revealed by random testing.
HOW DO I KNOW IF I AM ELIGIBLE FOR A VALSARTAN LAWSUIT?
If you took Valsartan and subsequently were diagnosed with cancer, or experienced other serious Valsartan side effects, then you may be eligible for a Valsartan lawsuit and possibly may be entitled to substantial compensation. Contact Valsartan Lawyer Timothy L. Miles today and see if you are eligible for a Valsartan lawsuit.
WHAT CAN A VALSARTAN LAWYER DO FOR ME?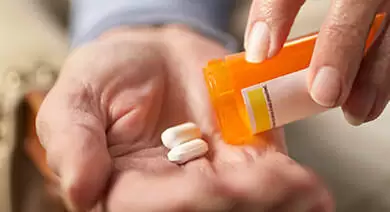 If you suffered serious Valsartan side effects after taking Valsartan, contact us to see if you meet the Valsartan lawsuit criteria If you suffered serious Valsartan side effects after taking Valsartan, contact us to see if you meet the Valsartan lawsuit criteria
While it is possible for individuals whom these products might have injured to go it alone without knowledgeable representation, those who do so run the risk of receiving an unfair settlement — if they get anything at all. Because drug makers have large amounts of money to spend on lawyers, going up against them as an individual can be intimidating and difficult. With an experienced Valsartan Lawyer, you can have the best chance to stand up to these large companies and optimize your chances of being fully compensated. Call Valsartan lawyer Timothy L. Miles today and see if you are eligible for a Valsartan lawsuit.
HOW DOES A VALSARTAN LAWSUIT WORK?
First, you Valsartan lawyer will confirm you are eligible for a Valsartan lawsuit. Before your Valsartan lawsuit is officially filed with the court, your Valsartan Lawyer will fully explain the process and will then need to ask you a few questions about your (or your family member’s) medical history, how long you have been taking Valsartan, and about your Valsartan side effects and they have affected your life.
Your Valsartan Lawyer will then draft what is known as a complaint. This will be a multi-page document explaining why the defendant is responsible for your injuries and what damages (that is, compensation) you are seeking. Once your complaint is filed with the court, your Valsartan lawsuit officially begins. From here, it will be a lot of back and forth between you Valsartan Lawyer and the attorney(s) for the defendant in an attempt to resolve the matter. The attorneys may take review documents, take depositions, issue subpoenas, hire experts, calculate damages, attend hearings, and file motions, briefs, evidence or other documents with the court during the stages of the lawsuit. If your Valsartan lawsuit is not dismissed and a settlement cannot be reached, the case will proceed to a jury trial. CALL A VALSARTAN LAWYER AND SEE IF YOU ARE ELIGIBLE FOR A VALSARTAN LAWSUIT
If you or a loved one has received a cancer diagnosis or experienced other serious Valsartan side effects after taking Valsartan, contact us for a free, no-risk case evaluation to see if you meet the Valsartan lawsuit criteria. We might be able to help you pursue compensation for the harm you and your loved ones have suffered if you meet the Valsartan lawsuit criteria and are eligible for a Valsartan lawsuit.
Call Valsartan Lawyer Timothy L. Miles today to see how he can help you.A Valsartan Lawyer can explain the process and answer any questions you may have including whether you meet the Valsartan lawsuit criteria. While there is still time to file a lawsuit, be mindful that these cases are time sensitive, and give us a call today. TIMOTHY L. MILES, ESQ.Timothy L. Miles is a nationally recognized shareholder and consumer rights attorney raised in Nashville, Tennessee. Mr. Miles was recently selected by Martindale-Hubbell® and ALM as a 2023 Top Ranked Lawyer, 2023 Top Rated Litigator. and a 2023 Elite Lawyer of the South. Mr. Miles also maintains the AV Preeminent Rating by Martindale-Hubbell®, their highest rating for both legal ability and ethics. Mr. Miles is a member of the prestigious Top 100 Civil Plaintiff Trial Lawyers: The National Trial Lawyers Association, a superb rated attorney by Avvo, a recipient of the Lifetime Achievement Award by Premier Lawyers of America (2019) and recognized as a Distinguished Lawyer, Recognizing Excellence in Securities Law, by Lawyers of Distinction (2019). Mr. Miles has published over sixty articles on various issues of the law, including class actions, whistleblower cases, products liability, and more. |
|
CONTACT
The Law Offices of Timothy L. Miles Tapestry at Brentwood Town Center 300 Centerview Dr., #247 Brentwood, TN 37027 Phone: (855) 846-6529 Email: [email protected] HOURS OF OPERATION Mon-Fri: 24/7 Sat-Sun: 24/7 |
CONNECt FacebooK
|
